For the past two centuries, humans have relied on fossil fuels for energy to concentrate; Hundreds of millions of years of photosynthesis crammed into a suitable, viable material. But this supply is limited and fossil fuel consumption has a major negative impact on the global climate. “The biggest challenge that many people don’t understand is that even nature doesn’t have a solution to the amount of energy we use,” said University of Chicago’s Wenbin Lin. Even photosynthesis isn’t good, he said: “We’re going to do better than (Fastest Artificial Photosynthesis system developed) nature, and that’s scary.”
One option scientists may be exploring is “artificial photosynthesis” – reprogramming plant processes to produce our own form of fuel. However, the one-sheet chemical properties are very complex and not easy to use for our own purposes.
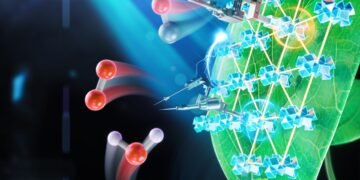
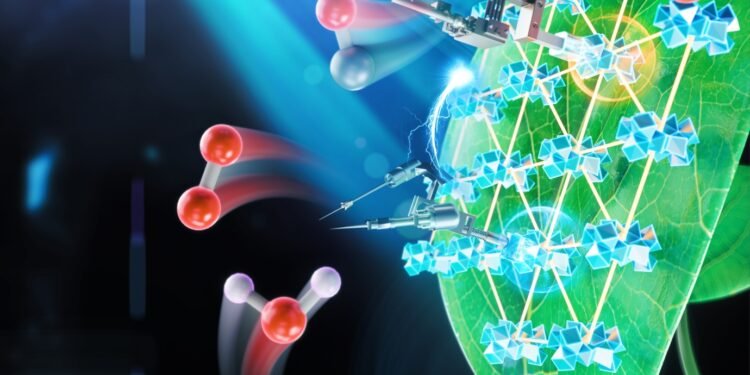
Nature Catalysis Research by six University of Chicago chemists shows a newly developed photosynthesis system that is orders of magnitude more productive than previous artificial systems. Unlike normal photosynthesis, which produces carbohydrates from carbon dioxide and water, artificial photosynthesis can produce ethanol, methane or other fuels.
Although it still has a long way to go before it becomes a way to power your car every day, the system gives scientists new directions to explore and could be useful in the future. short for the production of other chemical products.
“This is a big improvement over the current methods, but as important, we can show a clear understanding of how this system works at the molecular level, something that has never been done before ,” said Lin, who is a hard worker. James Franck Professor of Chemistry at the University of Chicago and lead author of the study.
“We’ll need something else”
“Without natural photosynthesis, we would not be here. It made the oxygen we breathe on Earth and makes the food we eat,” Lin said. “But it never worked well enough to give us fuel to drive; so we have to look for something else.
The problem is that photosynthesis was designed to produce carbohydrates, which are good for fueling us, but not for our cars, which require much more energy. Researchers who want to create an alternative to fossil fuels must rethink the process to produce more fuels, such as ethanol or methane.
In nature, photosynthesis occurs through many complex assemblies of proteins and pigments. They absorb water and carbon dioxide, tear apart molecules, and rearrange atoms to form carbohydrates, which are long chains of hydrogen-oxygen-carbon compounds. However, scientists need to restart the reaction instead of creating a different structure with only hydrogen surrounded by sweet carbon dioxide – CH4, also known as methane.
This re-engineering is much trickier than it looks; people have been dealing with it for decades, trying to get closer to natural performance.
Lin and his lab team thought they could try adding something that artificial photosynthesis systems haven’t incorporated to date: amino acids.
The group started with a type of material called a metallic organoframework, or MOF, a compound made up of metal ions held together by molecular bonds. Then they arranged the MOF as a single layer, to provide the highest surface area for chemical reactions, and put the whole thing in a solution that included a cobalt compound to transfer electrons. Finally, they added amino acids to the MOFs and tested to see which worked best.
They can improve two parts of the reaction: the process that separates water and that adds electrons and protons to carbon dioxide. In both cases, amino acids helped the reaction to proceed more efficiently.
However, even at high efficiency, artificial photosynthesis still has a long way to go before it can produce enough fuel to be necessary for commercial use. “Where we are now, it would have to increase by several orders of magnitude to produce enough methane for our consumption,” Lin said.
The reaction can be applied widely to other chemical reactions; A lot of fuel will be produced for this to have an effect, but a small amount of some particles, such as pharmaceuticals and nylon, and others, can be very useful. “Many of these basic processes are the same,” Lin said. “If you create the right chemicals, they can connect to many systems.”