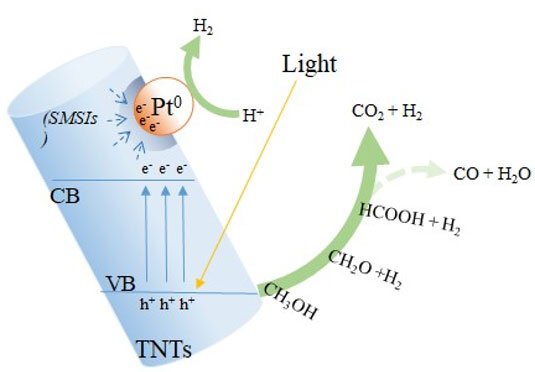
In a paper published in NANO, researchers from National Taiwan University examined the photocatalytic performances of titanate nanotubes (TNTs) against commonly-used titanium dioxide (TiO2) and discovered superior performance of TNTs.
In the study, TiO2 was used as a reference support compared with TNTs synthesized by a facile method. The results showed that Platinum (Pt/)TNTs fabricated using the microwave heating process enhanced the hydrogen evolution from methanol to a greater extent than Pt/TiO2. The high surface area of TNTs can improve adsorption of methanol on the active site and prevent the formation of agglomerated fine Pt particles.
Additionally, the high surface area led to an increased contact area between Pt and Ti atoms, which enhanced the strong metal-support interaction and increased H2 production performance. This is due to the absorption spectra of TNTs shifting toward the visible light region to a greater extent after loading Pt, thereby improving the selectivity of formic acid decomposition to CO2. Therefore, Pt/TNTs, which have considerably high photocatalytic efficiency, are viable in further applications as promising photocatalysts.
The titanate nanotubes (TNTs) composites enhanced the photocatalytic selectivity for H2 generation from formic acid better than Pt/TiO2. In addition, intensified electronic interactions occur between the components of TNTs and the Pt atoms in terms of the strong metal-support interaction, consequently influencing the behavior of photocatalysts. Therefore, the photocatalyst formed by Pt and TNTs has higher photocatalytic performance than TiO2 from a 20% v/v methanol solution under UV and visible light irradiation.
TNTs offer higher active surface area than TiO2 nanoparticles. The high surface area provides short diffusion paths for electrons and holes, prompting them to transfer to the surface and reducing the recombination of electrons and holes. Also, X-ray Photoelectron Spectroscopy (XPS) results of the paper showed negative shifts of the Pt binding energies and positive shifts of Ti binding energies due to the strong metal-support interaction between Pt and TNTs. Thus, the remarkably high photocatalytic efficiency of TNT composites facilitates their application as promising photocatalysts.
Besides, it is worth noting that one mole of HCOOH decomposes into one mole of CO2 and one mole of H2, or one mole of CO and one mole of H2O. Thus, it is important to increase the selectivity of formic acid decomposition for CO2 evolution. The results show bare TNTs and Pt/TNTs resulted in lower CO generation than bare TiO2 and Pt/TiO2. This result may be attributed to the inability of CO to diffuse into the pores of TNTs because of the diameter difference, because the kinetic diameter of CO (0.38 nm) is larger than that of CO2 (0.33 nm).
Will the different structure of the photocatalyst promote the photocatalytic selectivity of formic acid to H2? The researchers prove tubular TNTs composites enhanced the photocatalytic hydrogen generation better than TiO2.
































