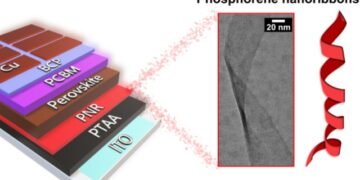
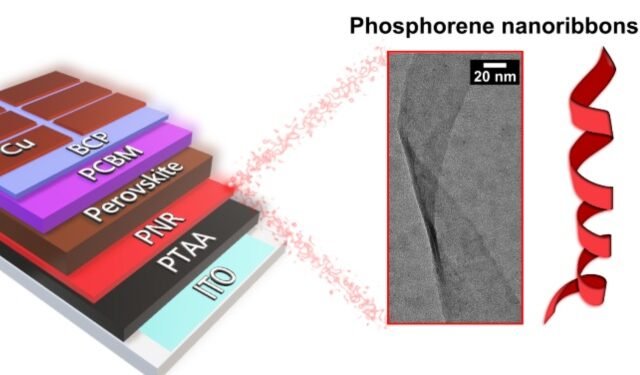
PNR is a ribbon-like (Thin ribbons can improve the batteries needed for clean transportation with solar power) 2D phosphorous fiber, which, like graphene, has a single layer of atoms. They were created in 2019 by a team of Professor Chris Howard of UCL following more than a hundred theoretical papers predicting that they will have many interesting and useful properties.
The PNR found their first solar cell-powered device in 2021, led by Dr Tom Macdonald of Queen Mary University of London/Imperial College London and supported by the UCL team. He showed that PNR can be printed as an additional layer to benefit the performance and efficiency of solar cells by improving the “space conversion”. “Spaces” are partners of electrons in electrical transport, therefore making them mobile (the measure of their speed through the material) helps to move electricity between levels efficiently.
Today, QMUL and UCL have teamed up again to provide a vision for how PNR can help solve the energy crisis.

Commenting on the new project (Thin ribbons can improve the batteries needed for clean transportation with solar power), Dr Macdonald, a researcher at Queen Mary University of London, said: ‘It is exciting as we consider how NRP can play an important strategic role in Our march against climate change. Last year, we showed that PNR can be printed on perovskite solar cells to improve their efficiency; and enable cheaper printing in thin, flexible films compared to non-flexible silicon-based solar cells. The promise of our PNR solar cells is amazing, but that’s just the beginning of many areas PNR can change, from lithium-ion batteries to clean hydrogen gas production.
From their Joule perspective, Dr. Macdonald outlines the key steps that researchers around the world have taken to develop and use PNR, including recent work showing that incorporating PNR into lithium-ion batteries improves performance and stability. better power, as the PNR can. to prevent the formation of unwanted dendrites that grow from the surface of the negative electrode. The dendrites can pierce the separator and enter the cathode of the battery. This causes electronic contact between the positive and negative electrodes, causing instability in lithium-ion batteries.