Japanese researchers are investigating the main cause of the fusion of elements with silver-containing super atoms and reveals Schematic representation of silver-based super atomic particles.
Super atomic particles containing noble metals such as gold and silver are being studied for their ability to bind to super atomic particles. However, the understanding of super atomic particles based on silver is not limited. To fill this gap, Japanese researchers studied two bimetallic super atomic molecules with silver as the main component to determine the main reason for their formation. It is hoped that their findings will promote (Schematic representation of silver-based super atomic particles) the development of new materials in the future.
In recent decades, metal nanoclusters composed of noble metals such as gold (Au) and silver (Ag) have attracted attention as super atoms for the synthesis of materials with unique properties and potential new applications. These super atoms (also called “artificial atoms”) are usually a few hundred atoms and exhibit different properties from their regular counterparts. However, like real atoms, the stability of these super atoms is determined by creating a closed electronic structure.
Ag-based super atoms are known for their superior properties and functions, including photoluminescence and selective catalytic activity, compared to those of Au-based super atoms. However, most research in this area has focused on Au-based super atomic molecules.
To fill this research gap, Japanese researchers studied the formation of superatomic molecules composed of Ag and investigated the factors involved in this formation. Professor Yuichi Negishi of Tokyo University of Science and Technology (TUS) led the study with input from Dr Sakiat Hossain of TUS, and Professor Tetsuya Taketsugu and Assistant Professor Takeshi Iwasa of Hokkaido University, and it was published in the journal Communications Chemistry in March 28. 2023.
Speaking about the motivation behind the study of Ag-based super atoms, Prof. Negishi says, “Until now, we humans have created many useful things from the things we have in the world. However, looking to the future and the energy is more complex and environmental problems, there is a need to create innovative products and services.”
For this, the researchers combined two di-super atomic atoms with bromine (Br) as a bond: ([Ag23Pt2(PPh3)10Br7]0 and [Ag23Pd2(PPh3)10Br7]0 (PPh3 = Triphenylphosphine). The first consists of two icosahedral Ag12Pt super atoms connected by sharing a vertex and a platinum (Pt) atom resides between the positions of each superatom. In contrast, the other super atomic molecule has two icosahedral structures Ag12Pd with palladium (Pd) as the central atom.
The geometric / electronic structure and stability of these two nanoclusters are analyzed and compared to [Ag23Pt2 (PPh3)10Cl7] 0 (1) and [Ag23Pd2 (PPh3)10Cl7]0 (2) – both nanoclusters show geometric similarities and synthesized nanoclusters. , which has chlorine (Cl) as the bonding atom.
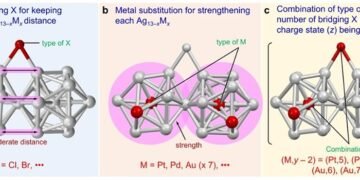
By examining the geometric structure of the four nanoclusters, the researchers observed an interconversion between two icosahedral structures with Br as the binding ligand. The researchers suggest that this rotation stabilizes the nanocluster by increasing the distance between the two icosahedral structures.
In addition, the large Br atom was found to introduce significant hindrance into the molecule, which both shifted the PPh3 molecule away from the long axis of the metal nanocluster and changed the long bond of Ag-P and Ag-Ag. job. These results show that although the type of halogen bonding slightly affects the geometric structure of the nanoclusters, it does not prevent their formation.
“The type of halogen bonding appears to have little effect on whether superatomic particles are formed, as long as the halogen bonding is large enough to maintain space distance between two Ag12M structures,” explains the teacher. Negishi.
However, the stability of the nanocluster depends on the number of halogens attached to it. Like atoms, stable metal nanoclusters require a filled valence shell. In the case of the prepared nanoclusters – which have a total of 16 valence electrons – the researchers were able to combine a maximum of five bridging halogens to hold the nanocluster in a stable or cationic state.
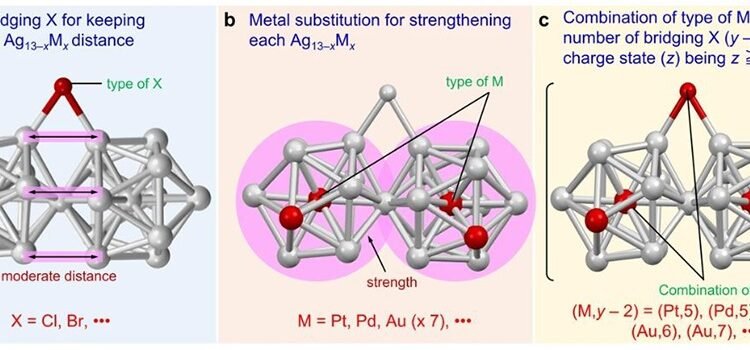
The presence of central Pd and Pt atoms was found due to the formation of metal nanoclusters. The replacement of the center of Ag13 by Pt or Pd led to an increase in the average binding energy in the nanoclusters, which makes it suitable for the formation of particles.
In general, the researchers identified three key points for creating and separating superatomic molecules consisting of two Ag13-xMx elements connected by vertex distribution. These include the presence of a bonding halogen that can maintain a good distance between the two structures, the combination of heteroatoms and bridging halogens that produce 16 valence electrons, and the structure of a strong icosahedral nucleus than Ag13.
According to Professor Negishi, “These findings provide clear design principles for creating molecular devices with different properties and functions, and can help solve clean and environmental problems.”
Source: Tokyo University of Science, Japan