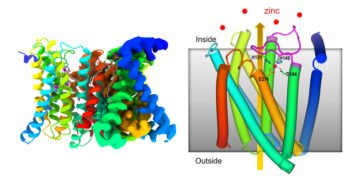
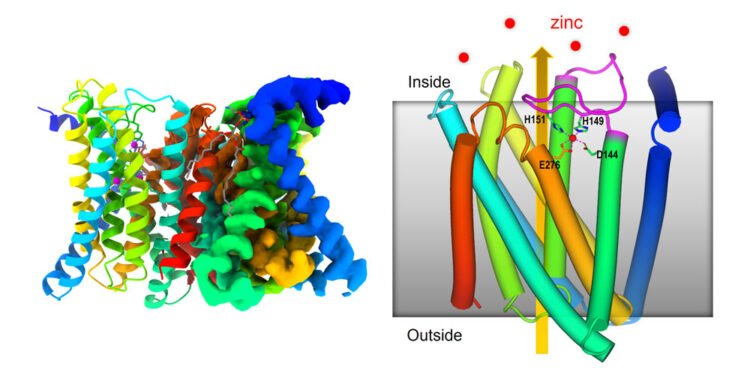
A new Cryo-EM structure of a zinc-transporting protein reveals how this molecular machine works to regulate cellular levels of zinc, an essential micronutrient i.e. The zinc transporter has a self-regulating sensor.
Scientists at the US Department of Energy (DOE) Brookhaven National Laboratory have determined the atomic structure of the protein that transports zinc, the molecular machine that controls the level of this important element in cells. As described in a paper published in Nature Communications, the structure reveals how the cell membrane protein changes shape to allow zinc from the environment into the cell, and temporarily blocks this automatically when the level of zinc inside the cell is high.
“Zinc is essential for many biological activities, but excess can be a problem,” said Qun Liu, a Brookhaven biophysicist who led the project. “Over the course of evolution, species have evolved in many ways to manage zinc. But no one has shown that the transporter that controls zinc from the environment can regulate its own activity. Our study is the first to demonstrate a zinc transporter with such a sensitivity.
The research was conducted as part of the Quantitative Plant Sciences Initiative (QPSI) at Brookhaven Lab. By using a bacterial model of a zinc transporter that shares important characteristics with zinc transporters in plants, scientists gained important insight into how these proteins work.
“This research is part of our efforts to understand how plants produce nutrients such as zinc so that we can understand how to develop plants that are able to grow in the soil for bioenergy production,” said John, department of Brookhaven Laboratory Shanklin, novelist.
The findings may also suggest ways to engineer zinc-rich crops to improve their nutritional value, the scientists said.
Cryo-EM plus calcns
To resolve the structure of the protein, the Brookhaven team used cryo-electron microscopy (cryo-EM) at the Laboratory of Biomolecular Structure (LBMS). With this method, scientists can measure many different variants of a protein instead of a single crystallized form. This is important because, in nature, protein is dynamic, not static. their pieces are moving.
“Cryo-EM does not require proteins to form crystals, so we can capture complex structures that may not be possible using X-ray crystallography, another technique for studying protein structure,” Liu said. “Basically, with cryo-EM, we can capture many frames of the ‘movie’ to obtain a very useful method for understanding the biological functions of proteins.”
To resolve the many structural differences, scientists need powerful computing tools. These include artificial intelligence systems that use machine learning, some of which were developed by Liu. Using these algorithms, scientists can automatically select half and process millions of cryo-EM images to find clusters of similar structures. The technique allows them to achieve the highest possible resolution, thus revealing the details of atomic structures.
For this study, this cryo-EM approach revealed the main steps of the zinc transporter ZIP (Zrt-/Irt-like protein) which reveals how it regulates its own zinc uptake activity based on the amount zinc is present in it cells.
“Our new data allowed us to rethink previous ideas about how this protein works,” Liu said.
Turn to enter, mind to stop
Previous reports based on X-ray crystallography and coevolutionary analysis suggested that the carrier may function as a kind of “lift” to transport zinc. This new research shows how the interaction with zinc on both sides of the cell membrane stimulates the movement of parts of the protein to bring zinc into the cell and, importantly, prevent its absorption when the level inside is high.
Liu said, “The main point of our system is to show that when the level of zinc inside the cell reaches a certain level – beyond what is needed to meet the needs of the cell – the excess zinc binds to the membrane. ,” said Liu. “Then, as this flexible loop repeats itself, it folds back on itself and binds in a way that prevents zinc from entering the cell.”
“The lock almost went into the shower and blocked it,” Shanklin added.
The scientists also discovered how other parts of the protein move to absorb zinc.
When the level of zinc in the cell is low, the zinc falls to its side and the plug comes out of the carrier. Zinc from the environment can enter the carrier. In the transporter, zinc causes parts of the protein machine to rise and fall, closing the exit to the remote environment. Once the zinc enters the cell, the machine resets to work again.
“Our cryo-EM model is the first to show how the loop domain of a protein changes transporter activity in response as a function of zinc levels,” said Liu.
It is also the first structure to show that this zinc carrier is a structure of two identical proteins, known as dimers. “It takes two molecules to do the job,” Liu said.
Scientists believe that having two molecules that function as a dimer can bind to its activity or stability, which they will use future computer simulations to determine how the molecules how to work together.
“This research may lead to new ways of making zinc transporters and microbes in plants to increase their growth in conditions where zinc is very low or high, which may be in remote soils for production of bioenergy and bioproducts.” Liu said.
Source: Brookhaven National Laboratory.