Scientists have developed two new materials assembled from silver clusters with excellent stability and the ability to detect Fe3+ selectivity. Silver cluster composites (SCAM) are emerging catalysts with molecular design capabilities and unique properties. However, due to their unique architecture and different solvents, their widespread applications are still limited.
Now, researchers at Tokyo University of Science in Japan have produced two SCAMs that exhibit excellent fluorescence and high sensitivity to Fe3+ ions in aqueous solutions, demonstrating their potential for environmental monitoring and research.
In recent years, there has been an increasing interest in the use of silver nanoclusters (Ag NC), nanoscale silver particles containing tens to hundreds of atoms, in various fields such as material science, chemistry and biology.
Ag NCs generally have sizes ranging from 1 to 3 nm. Scientists have made great progress in creating and manipulating Ag NCs, leading to the development of silver cluster composites (SCAM). SCAM is a virtual light source consisting of several Ag NCs connected together, connected to special binding molecules called \”ligands\”.
What makes them different is their ability to create things at the molecular level and their unique photophysical properties. However, their widespread use has been limited due to their different formation patterns when immersed in different solvents.
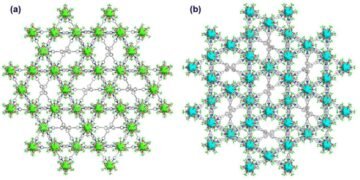
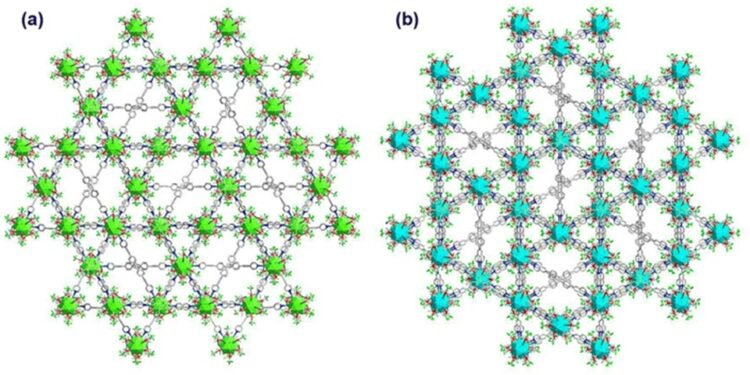
To solve this problem, a team of researchers from Tokyo University of Science (TUS), led by Professor Yuichi Negishi and including Assistant Prof. Saikat Das, recently developed two new three-dimensional luminescent SCAMs (4.6) with a core of Ag12.
Cluster connected by quadridentate pyridine linkers – [Ag12 (StBu) 6 (CF3COO) 6 (TPEPE) 6] n, means TUS 1 and [Ag12 (StBu) 6 (CF3COO) 6 (TPVPE) 6] n, shown TUS 2. “We have successfully designed two buildings connected to the Silver cluster with a new connection system, which can be applied to environmental monitoring and evaluation,” said Prof. Negishi.
The study was published in the journal Nanoscale on June 26, 2023. The researchers synthesized SCAM using the same simple reaction method, the only difference being the binding molecules. They combined [AgStBu]n with CF3COOAg in a solution of acetonitrile and ethanol. Molecular formula TPEPE = 1,1,2,2-tetrakis(4-(pyridin-4-ylethynyl)phenyl)ethene and TPVPE = 1,1,2,2-tetrakis(4-((E))pyridin-4yl) vinyl)phenyl)ethene dissolved in different chemicals, namely tetrahydrofuran and dichloromethane, respectively.
The metal solution is added to the bonding molecule solution and allowed to glow in the dark. After one day, yellow crystals form near the junction of the two solutions, indicating the production of SCAM.
The team conducted various tests to evaluate the structure of SCAM. They found that TUS 1 has a rod-like structure, while TUS 2 has an inhibitory structure.
They also tested the chemical stability of the materials by placing them in different solvents, and found that their crystal structure did not change, demonstrating their exceptional stability. In addition, due to their unique fluorescence properties with a quantum efficiency of up to 9.7% and stability in water, the group investigated the ability of SCAM to detect metal ions in aqueous solutions.
In their excitation, both SCAMs were highly sensitive to Fe3+ ions, which closed their lights well at room temperature, indicating the presence of Fe3+ ions. The limit of detection for Fe3+ ion was 0.05 and 0.86 nM·L-1 for TUS 1 and TUS 2, respectively, compared to standard values.
Moreover, both SCAMs were highly selective towards Fe3+ and other metal ions were unaffected. These results show apps that are used in the environment, mainly in FERSE Fe3 + in water. “It can connect the silver of silver type that may allow to create different things that have different phycochemical items.
Further developments in nanotechnology may thus allow us to manufacture materials and devices on a smaller scale, which should lead to higher performance materials and devices,” says Professor Negishi. Hopefully this technology will lead to more nanomaterials for environmental research and other innovative applications!
Source: Tokyo University of Science