Reinventing quantum dot production through flow chemistry and sustainable technologies
In a context where the demand for innovative materials continues to grow, particularly to meet current technological and environmental challenges, research on nanomaterials is emerging as a strategic field. Among these, quantum dots are attracting particular attention due to their unique properties and vast range of applications. A team of researchers from the University of Liège recently distinguished itself by proposing a step towards a more sustainable production of these nanostructures.
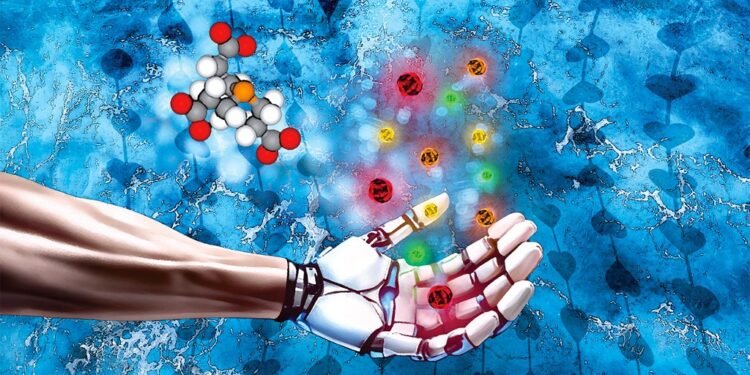
Quantum dots (QDs) are nanometer-sized semiconductor nanoparticles with unique optical and electronic properties. Their ability to absorb and emit light with high precision makes them materials of choice for solar cells, LEDs, medical imaging, and sensors. In a new study, researchers at ULiège have developed the first intensified process compatible with industrial scale production to produce cadmium chalcogenide QDs (semiconductor compounds very useful in optoelectronics and nanotechnologies) in an aqueous medium, from a new biocompatible source of chalcogens (chemical elements such as sulfur, selenium, and tellurium). Unlike traditional methods, which rely on organic solvents, this flow process relies on water and offers a unique combination of sustainability, safety, and flexibility—a major step toward responsible production of advanced nanomaterials.
A collaboration between two ULiège laboratories – CiTOS (Centre for Integrated Technology and Organic Synthesis) and the MSLab – has led to the design of an original water-soluble source of chalcogens and an integrated flow process capable of producing high-quality QDs, compatible with biomedical applications. These results were published in Chemical Science , while a broader review on the sustainable production of quantum dots was recently published in Materials Science and Engineering R. “ The idea came to us from peptide synthesis, where TCEP is a well-known water-soluble reducing agent ,” explains Jean-Christophe Monbaliu, director of CiTOS. “ We saw a unique opportunity to make it a safer, stable and easily transposable chalcogen transfer agent for large-scale production – this idea proved very promising .”
To better understand the interactions between TCEP and chalcogens (sulfur, selenium, tellurium), the CiTOS team partnered with Cédric Malherbe (MSLab), a specialist in vibrational spectroscopy. Using in situ Raman spectroscopy , they were able to follow the reaction mechanisms and dissect, step by step, the specificity of the mechanism—an approach that is still rare in this field. “ Thanks to this collaboration, we were able to capitalize on cutting-edge analytical tools to observe the reactions live ,” emphasizes Cédric Malherbe, “which remains relatively marginal in this field. ”
The system developed not only increases productivity, but also drastically reduces waste, energy consumption and purification steps. “Even though cadmium-based QDs are very efficient, their toxicity remains problematic, especially with increasingly strict environmental standards,” recalls Carlotta Campalani, researcher at CiTOS. “We are exploring greener and less toxic alternatives, while maintaining high performance .
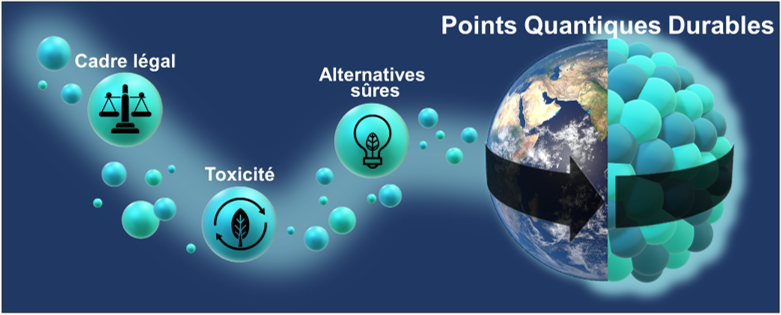
This research offers a realistic and responsible path towards the production of nanomaterials on an industrial scale, and reflects ULiège’s commitment to innovation, at the crossroads of chemistry, sustainability and the technologies of tomorrow.
“Source: University of Liège