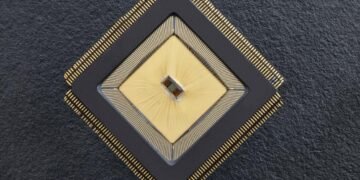
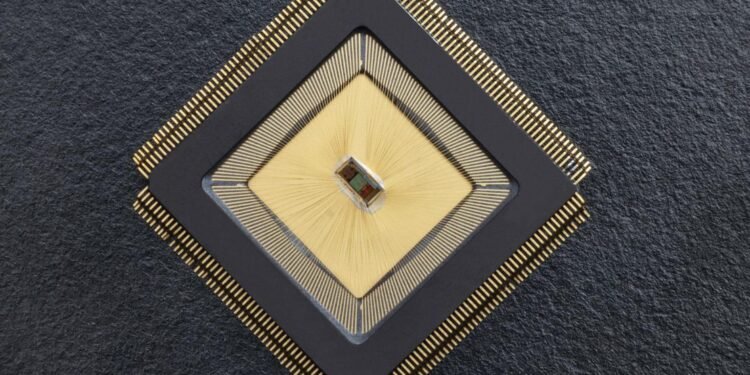
Mahsa Shoaran from the School of Engineering Integrated Neurotechnologies Laboratory collaborated with Stéphanie Lacour from the Soft Bioelectronic Interfaces Laboratory to create NeuralTree: a closed neuromodulation system that can detect and reduce (A neurochip to manage brain disorders) disease symptoms. Using a high-performance 256-channel detection system and a dynamic machine learning system, the system can extract and classify multiple biomarkers from real patient data and in vivo animal models of disease, leading to high level of accuracy and reporting symptoms.
“NeuralTree benefits from the accuracy of neural networks and the hardware performance of decision tree algorithms,” says Shoaran. “This is the first time that we have been able to integrate such a complex, yet functional, neural system for binary processing tasks, such as grasping or vibration detection, and many various functions. such as coordination of finger movements. for neuroprosthetic applications.”
Their results were presented at the IEEE 2022 International Conference on Solid-State Circuits and published in the IEEE Journal of Solid-State Circuits, the flagship journal of the integrated circuit community.
Performance, scalability and versatility
NeuralTree works by extracting neural signals — patterns of electrical signals known to be associated with certain neurological disorders — from brain waves. It separates the symptoms and shows whether they indicate an impending epileptic seizure or a parkinsonian tremor, for example. If a symptom is detected, the neurostimulator – which is also in the chip – works, sending electrical impulses to prevent it.
Shoaran explains that NeuralTree’s unique features give the system unprecedented performance and flexibility compared to the state of the art. The chip has 256 input channels, compared to 32 for previous embedded devices, allowing for higher input data processing. The area-saving design of the chip means it is also small (3.48mm2), giving it great potential for scalability to other channels. The integration of “power-aware” learning algorithms – which predict dynamic features – also makes NeuralTree more efficient.
In addition to these advantages, the system can detect different types of symptoms than other devices, which until now have focused mainly on detecting epileptic seizures. The chip’s machine algorithm was trained on processing data from patients with epilepsy and Parkinson’s disease, and correctly classified previously recorded nerve signals from the two domains.
“To our knowledge, this is the first demonstration of Parkinsonian tremor detection with an on-chip processor,” Shoaran says.
The algorithm is self-updating
Shoaran is interested in making the nerve cells more efficient to make disease management more effective, and he is considering new innovations.
“Ultimately, we can use neural networks for many different problems, and we need algorithmic thinking and advances in chip design to do that. This work is highly interactive and therefore requires collaboration with laboratories such as the Soft Bioelectronic Interfaces Laboratory, which can develop technological electrodes, or laboratories with access to high-quality patient data.
As a next step, it wants to update the on-chip algorithmic update to follow the origin of the nerve signal.
“Neural signals change, so over time, the efficiency of the neural interface will decrease. We are always trying to make other algorithms more accurate and reliable, and one way to do that would be to enable on-chip updates, or algorithms that can update themselves.
Source: EPFL