MIT engineers are discovering that special nanoparticles can isolate proteins quickly and inexpensively in a bioreactor, A new purification technique could make protein drugs cheaper.
One of the most expensive (A new purification technique to make protein drugs cheaper) processes in the production of protein drugs such as antibodies or insulin is the purification process: separating the protein from the bioreactor used to produce it. This step can represent up to half of the total cost of protein production.
In an effort to help reduce these costs, engineers at MIT have developed a new way to do this type of cleaning. Their approach, which uses special nanoparticles to make proteins faster, can help make protein drugs more affordable and accessible, especially in developing countries.
“This work uses bioconjugate-functionalized nanoparticles that act as a template to increase protein crystal stability in small concentrations,” says Kripa Varanasi, professor of mechanical engineering at MIT and lead author of the new study. “The goal is to reduce costs so that this type of drug production becomes affordable in developing countries.”
The researchers showed that their method can be used to use lysozyme crystals (an antimicrobial enzyme) and insulin. They believe that it can be added to many other useful proteins, including vaccines and vaccines. MIT graduate student Caroline McCue led the study, which appears today in the journal ACS Applied Materials and Interfaces. Henri-Louis Girard PhD ’20 is also the author of the article.
Pure protein
Antibiotics and other protein-based drugs are part of a growing group of drugs known as biomolecules, which also include molecules such as DNA and RNA, as well as cell-based therapies. Many protein drugs are produced by living organisms such as yeast in large bioreactors.
Once these proteins are produced, they must be separated in the reactor, which is usually done through a process called chromatography. Chromatography, which separates proteins based on their size, requires special equipment that makes the process expensive.
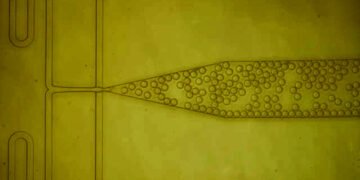
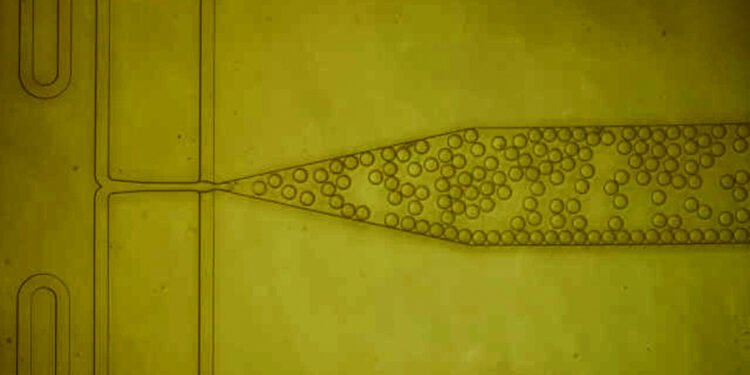
Varanasi and his colleagues decided to try a different approach, based on protein crystals. Researchers often use protein crystals to study their structure, but this method is considered too fast for industrial use and does not work well with low protein concentrations. To overcome these obstacles, the Varanasi laboratory decided to use nanoscale structures to accelerate crystallization.
In previous work, the lab has used nanoscale designs to create water-repellent or modified surfaces for injecting highly viscous antibiotics. In this case, the researchers want to shape the nanoparticles so that they can increase the amount of protein on the surface and provide a matrix that will allow the proteins to adapt well and form crystals.
To create the surface they need, the researchers coated the gold nanoparticles with molecules called bioconjugates, substances that can help make connections between other molecules. For this study, the researchers used bioconjugates called maleimide and NHS, which are commonly used to tag proteins for research purposes or to add protein drugs to drug delivery nanoparticles.
Two views of circular particles moving horizontally at the top and bottom of the gif. Looking at the surface, small white spots quickly form on the material.
Looking down, the point takes a long time to come out. When the protein solution is exposed to these coated nanoparticles, the proteins accumulate on the surface and combine with bioconjugates. In addition, bioconjugates force proteins to conform in the same way, creating barriers for other proteins to join the crystal.
The researchers presented their approach to lysozyme, a well-studied enzyme that forms crystals, and insulin. They say it can be added to many other proteins.
“This is a general process that can also be replicated in other processes. If you know the protein structure you want to cut, you can add the right bioconjugates that will force the process to happen,” Varanasi says.
Rapid crystallization
In their study of lysozyme and insulin, the researchers found that the crystals formed faster when the protein-coated nanoparticles covered the bioconjugate, compared to free nanoparticles or no nanoparticles. With these coatings, the researchers found a seven-fold reduction in the initiation time – the time it takes for crystals to start forming – and a three-fold increase in the nucleation rate, i.e. the rate at which crystals grow spontaneously began.
“Even at low protein concentrations, we see many other crystals that use nanoparticles to bioconjugate,” McCue says. “Functionalized nanoparticles significantly reduce the time of insertion because these bioconjugates provide specific sites for protein binding. And because the nutrients bind together, they can form crystals quickly.
Additionally, the team used machine learning to analyze thousands of crystal images. “Protein crystallization is a stochastic process, so we need a large data set to be able to really measure whether our method optimizes the incubation time and the nucleation rate of the crystallization. “With so many images to process, machine learning is the best way to detect when crystals are forming in each image without having to manually go and count them,” says McCue.
The project is part of the Bill and Melinda Gates Foundation’s efforts to develop biological drugs, such as vaccines that have been shown to prevent malaria in clinical trials, which are common in developing countries.
The MIT team is currently working on improving this process so that it can be used in an industrial bioreactor to demonstrate that it can work with monoclonal antibodies, antibodies and other useful proteins.
“If we can make it easy to make these proteins anywhere, then everyone in the world can benefit,” says Varanasi. “We’re not saying it will be fixed tomorrow thanks to us, but it’s a small step that can help in this project.”