Researchers from the Max Planck Institute for the Science of Light (MPL) and the Max Planck Center for Physics and Medicine (MPZPM) in Erlangen represent a significant step forward in the identification of nanoparticles. They use a special microscopic technique based on interferreometry to rewrite existing instruments. One of the possible uses of this technique is the identification of diseases, A new way to explore the nanoworld.
A new way to explore the nanoworld. Nanoparticles are present in our body as protein aggregatives, lipid vesicles or viruses
Nanoparticles are everywhere. They are present in our body as protein aggregates, lipid vesicles or viruses. They are in our drinking water in the form of impurities. They are in the air we breathe like dirt. Many drugs are based on the supply of nanoparticles, including faxes that have just been delivered to us. Following the pandemics, the rapid tests used to detect SARS-Cov-2 were also based on nanoparticles. The red line we check every day contains many gold nanoparticles (A new way to explore the nanoworld) coated with egg white antibodies that report infection.
Technically, an object is called a nanoparticle if its size (diameter) is less than one micrometer (thousandth of a millimeter). Objects in the micrometer range can still be measured with a conventional microscope, but particles smaller, such as less than 0.2 micrometers, may be more difficult to measure or identify. Interestingly, it is also a measure of virus size, which can be as small as 0.02 micrometers.
Over the years, scientists and engineers have developed many tools for identifying nanoparticles. Basically, it is about measuring their concentration, checking their size and size distribution and determining their composition. A top example is the electron microscope. However, this technology has many disadvantages. It was very large and expensive and the studies took too long because the samples had to be carefully prepared and placed in a vacuum. And yet it is still difficult to visually determine the nature of the particles with an electron microscope.
The fast, reliable, lightweight and portable device that can be used in the office or in the field has a huge impact. Several optical instruments on the market offer such solutions, but their resolution and accuracy are not sufficient for the analysis of small nanoparticles, for example smaller than 0.1 micrometer (or otherwise 100 nm).
A team of researchers from the Max Planck Institute for Light Science and the Max Planck Center for Physics and Medicine have now invented a new tool that offers a huge leap in nanoparticle identification. The method is called iNTA, short for Interferometric Nanoparticle Tracking Analysis. Their results were published in the May issue of the internationally renowned journal Nature Methods.
Courtesy: Max Planck Institute for Light Science.
The method (A new way to explore the nanoworld) is based on interferometric detection of light scattering on individual nanoparticles traveling in a liquid. In such an environment, thermal energy constantly moves the particles in any direction. It turns out that the space explored by a particle at a given time is related to its size. In other words, small particles move “faster” and cover a larger volume than large particles. The equation that describes this phenomenon – the Stokes-Einstein relation – began at the beginning of the last century and has since been found to be used in many applications. In short, if one could trace a nanoparticle and gather statistics about its trajectory of nervousness, one could imagine its magnitude. So the challenge is to make very fast movies with running small particles.
Over the last two decades, MPL researchers have developed a special microscopy technique known as interferometric scattering microscopy (iSCAT). This technique is more sensitive to the identification of nanoparticles. By applying iSCAT to the problem of nanoparticle scattering, the MPL team realized that it could outperform existing tools in the market. The new technology has particular difficulties in decrypting mixtures of nanoparticles with different sizes and different materials.
There are many applications of the new method. A particularly exciting number of applications have to do with nanoscale-separating cars, the so-called extracellular vesicle. It is made from a lipid coating, such as nano soap foam. But the shell and the internal fluid also contain egg whites, which indicate the origin of the vesicles, ie. from any organ or cellular process. If the amount of egg white and / or the size of the sac deviates from the normal range, a person may become ill. Therefore, it is very important to find ways to identify extracellular v that scientists around the world can reap the benefits of iNTA.
esicles.
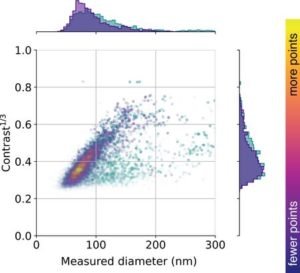
MPL and MPZPM researchers are now working to create a state-of-the-art system soFigure 2: This figure shows the distribution of vesicles collected from the urine of a healthy person as a function of vesicle size and iSCAT contrast (i.e., how fast they disperse in light). Researchers are currently investigating such distributions along with various diseases. Courtesy: Max Planck Institute for Light Science.