Although catalytic converters for passenger vehicles have been mandatory for over 30 years, there is still a long way to go. For example, they only work properly (Cleaner air with a cool catalytic converter) when the engine is warm enough, which is not always the case, especially with hybrid vehicles.
Researchers from the Eindhoven University of Technology (TU/e), together with colleagues from the University of Antwerp, have developed an air purifier that can effectively clean the air even in the heat. Their work was published in the journal Science on June 16.
The so-called three-way catalytic converter in the car’s air system is very expensive and works best when the exhaust gas has a temperature of several hundred degrees Celsius.
Therefore, when you start your car, or when you drive a hybrid car with a petrol engine and an electric motor that switches between driving, the gases that come out of the exhaust usually contain carbon monoxide and – deadly.
In a new Science paper, scientists led by Emiel Hensen now show that by changing the catalyst, toxic carbon monoxide can be completely converted to carbon dioxide even at high temperatures.
Very important
Automotive alloys are made by embedding precious metals such as platinum, palladium and rhodium into grains of cerium oxide, also known as cerium oxide. However, fine jewelry is rare and expensive.
Therefore, researchers around the world are working on ways to achieve the same or even better catalytic performance using these small materials. For example, in a previous paper, Hensen’s team at TU/e showed that by dispersing a noble metal in the form of a single atom, one leads not only to a reduction in material consumption, but to under certain conditions, the material makes it higher. and work more efficiently.
New size check
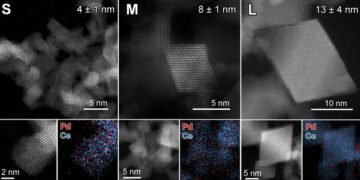
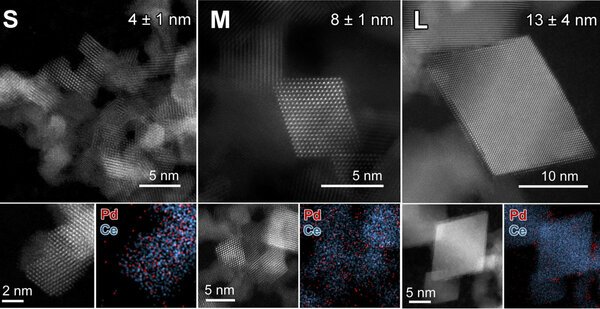
In the research project of the doctoral Valery Muravev, the researchers turned their attention from the noble metal to the underlying support material (cerium in this case) to improve the catalyst properties. They created cerium oxide in different crystal forms and placed the noble metals as single atoms in the same step.
They then investigated how well these compounds could combine oxygen atoms with carbon monoxide. Small 4 nanometer ceria crystals have been shown to improve the performance of palladium metal under cold start conditions in the presence of excess carbon monoxide.
This better performance can be explained by the higher reactivity of oxygen atoms and the smaller size of cerium oxide crystals. Under more common conditions, 8 nanometers have been found to be the optimal size of ceria crystals required to achieve high catalytic activity at temperatures below 100 degrees Celsius.
Wide range
This study shows that for the first time when developing, it pays to not only focus on the good metals that should do the job. In this case, the diversity of the working materials as a support for the working materials provides new and interesting opportunities to improve the materials that are produced in them, to improve the performance and specific chemical reactions.
This is also important for the development of a process to combine carbon dioxide from the atmosphere with green hydrogen to create fuel or compounds for the production of sustainable plastics. In collaboration with the British company Johnson Matthey, which produces catalysts for the automotive industry, the researchers will now further investigate how to translate this research into new products.
Source: Eindhoven University of Technology.