A catalyst, whose properties change in response to external factors such as light and heat, is being studied in many areas as a next-generation energy source. In particular, photosensitive materials attract a lot of attention because their physicochemical (Discovery captures the key concepts of crystal photochemistry!) properties can be modulated remotely without any physical contact.
As one of the photodetectors, photomechanical molecular crystals, which contain molecules that undergo photochemical reactions upon exposure to light, are being investigated for their unique photographic properties.
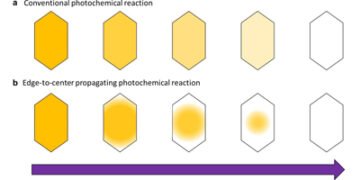
Unlike a solution where the molecules are independent, the molecules are dense and arranged in crystals, so different factors must be taken into account. special photoreaction in the crystal. However, previous studies of photoreactive molecular crystalline materials usually assume that the photoreactions in the crystal are continuous as solutions and follow the classical photoreaction kinetics. Therefore, understanding the changes of physicochemical properties based on photochemical reaction kinetics in crystals has become a problem for the further development of this research field.
A research group by Kohei Morimoto, who completed three years of doctoral studies at the Osaka University Graduate School of Engineering and Dr. Daichi Kitagawa and Professor Seiya Kobatake of Osaka University graduated in Engineering. Distyrylpyrazine (DSP) crystals spread from each side of the crystal toward its center. The results of this research were published online in Angewandte Chemie International Edition on November 3, 2022.
Normally, when light shines uniformly on the crystal, the color change caused by the photoreaction also occurs uniformly. However, the same study found that in the DSP crystal, the color change started at the edge of the crystal and continued in the middle, propagating the photoreaction as a wave. The same study determined that this spreading around the edges of the color change occurs due to two factors: the surface effect, which accelerates the reaction at the edge of the crystal, and the reinforcement effect, which also increases the reactivity of the crystal. crystal. neighboring particles have changed color.
“This activity, which comes from the concept of photochemistry, is very important in how we understand the basic science of photoreactions,” said Dr Kitagawa. “In the future, we would like to define the conditions necessary for this unique photoreaction to take place and find out how they can create new functional materials that take advantage of this opportunity.”