The new nanomaterial (improved hydrogen fuel production) will help extract hydrogen from the liquid energy carrier, which is an important step towards a stable and clean fuel source.
Hydrogen is a sustainable source of clean energy that prevents toxic emissions and adds value to many sectors of the economy, including transport, energy production, metal production and more. Hydrogen storage and transport technologies bridge the gap between renewable energy production and fuel consumption and are therefore an essential part of a viable hydrogen economy. But the traditional method of storage and transport is expensive and easy to pollute. As a result, researchers are looking for alternative techniques that are reliable, inexpensive, and simple. More efficient hydrogen supply systems can benefit from many applications, such as stationary power, portable power and the mobile automotive industry.
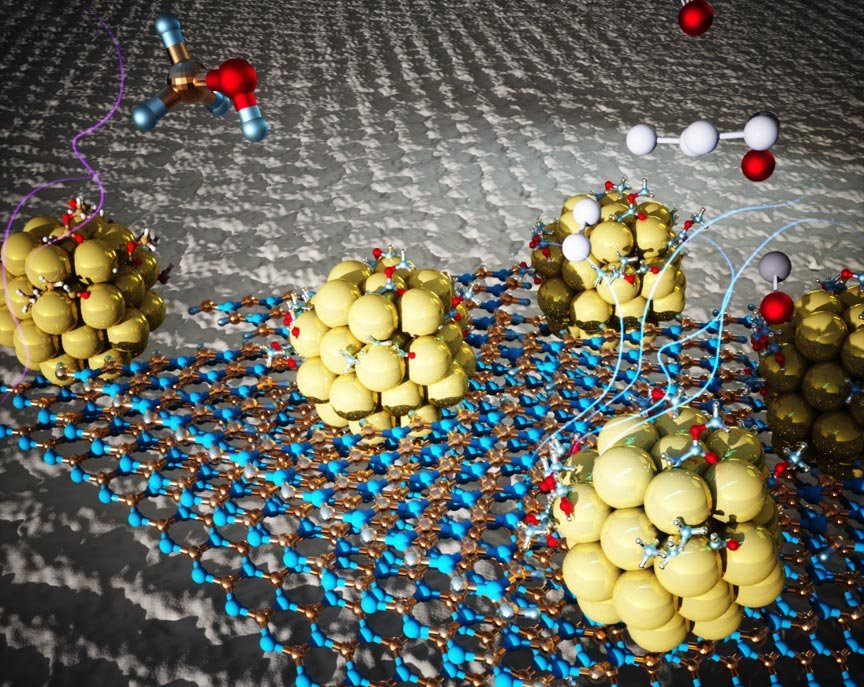
Now, as reported in the Proceedings of the National Academy of Sciences, researchers are designing and synthesizing an efficient material (improved hydrogen fuel production) to accelerate one of the limited steps in extracting hydrogen from alcohols. The material, the catalyst, is made of small pieces of metallic nickel anchored to a 2D substrate. A group led by researchers at Lawrence Berkeley National Laboratories (Berkeley Lab) Molecular Foundry found that the catalyst could be clean and effective in accelerating the reaction by removing hydrogen atoms from a liquid chemical support. The material is stronger and made of metals rich in the Earth than current options made of precious metals and helps hydrogen become a viable source of energy for a wide range. “We are not only presenting a higher activity catalyst than the other nickel catalysts we have tested as the main fuel for renewable energy, but also a broader strategy for using low-cost metals. In a wide range of responses,” said Jeff Urban, Molecular Foundry The research is part of the Hydrogen Materials Advanced Research Consortium (HyMARC), a consortium funded by the United States Department of Energy and Renewable Energy, Office of Hydrogen and Fuel Cell Technologies (EERE), through which five national laboratories are working to The results of this work (improved hydrogen fuel production) are fed directly into EERE’s H2 @ Scale vision for cost-effective production, storage, distribution and use in many sectors of the economy.
Chemical compounds that act as catalysts, such as those made by Urban and his team, are often used to increase the rate of a chemical reaction without depleting the compound itself – it can hold a particular molecule in a fixed position or act as an intermediary for an important reliable step. which needs to be completed. For chemical reactions that produce hydrogen from liquid supports, the most efficient catalysts are made of precious metals. However, these catalysts are associated with high cost and small amounts and are easily contaminated. Some cheaper catalysts made from more common metals tend to be less efficient and less durable, which limits their operation and their practical commitment to hydrogen companies.
Urban and colleagues have changed their strategy to target small, uniform nickel metal clusters to improve the performance and durability of these multi-earth metal catalysts. Small clumps are important because they increase the exposure of the reactive surface to a given amount of material. But they also tend to accumulate, which hinders their activity.
Postdoctoral research assistant Zhuolei Zhang and project scientist Ji Su, both Molecular Foundry and co-authors of the article, designed and conducted an anti-coagulation experiment by depositing 1.5 nanometer-sized nickel clusters in a 2D substrate made of boron and nitrogen designed to host a grid atomic dimples. The nickel clusters were evenly spaced and firmly anchored to the dimples. This design not only prevents agglomeration, but its thermal and chemical properties significantly improve the overall performance of the catalyst by direct contact with nickel agglomerates.
“The role of the substrate surface in the clustering and deposition phase has been found to be critical and can provide guidance for understanding its role in other processes,” says Urban. Detailed X-ray measurements and spectroscopy in combination with theoretical calculations show a lot about the underlying surfaces and their role in catalysis. Using Advanced Light Source tools, the DOE’s Berkeley Lab installation, and computational modeling methods, the researchers identified changes in the physical and chemical properties of 2D sheets as small nickel clusters evolved. adjacent edges. , which keeps the size of the clusters small. Small, strong clumps accelerate the processes in which hydrogen separates from its liquid support, giving the catalyst excellent selectivity, productivity and stable performance.
Calculations show that the size of the catalyst is the reason why its activity is one of the most closely related to other recently published ones. David Prendergast, director of the Molecular Foundry Theory of Nanostructured Materials Theory, and postdoctoral research assistant and co-author Anna Sanz-Matias used models and computational methods to discover unique geometric and electronic structures. in small metal clusters. Bare metal atoms, which are abundant in these small clusters, attract the liquid carrier more easily than larger metal particles. These exposed atoms also facilitate the steps of the chemical reaction, which removes hydrogen from the support, while preventing the formation of contaminants that can block the cluster surface. The material thus remains free of contamination during the important hydrogen production reaction steps. These catalytic and anti-pollution properties arose from imperfections intentionally embedded in 2D sheets and ultimately helped to reduce the size of the cluster.
“Pollution may not make worthless metal catalysts sustainable. Our platform opens new doors to system technology here, ”said Urban.
With their catalyst, the researchers achieved the goal of creating a relatively inexpensive, easy-to-use and durable material that would help remove hydrogen from liquid carriers for use as fuel.