Microstructurally Engineered Ultra-hard Diamond Possess Remarkable Toughness: By Dr Yashwant R Mahajan
Materials with superior hardness can be categorized as ultra-hard (Vickers hardness, Hv ≥ 80 GPa) and superhard (Hv ≥ 40 GPa) materials (Super-hard Materials: Advances in the Search and Synthesis of New Materials). Diamond, cubic boron nitride (c-BN) and cubic BC2N are considered to be ultra-hard with hardness values ranging from 70 -110, 45- 60 GPa(Super-hard Materials) and 75 GPa,(Mechanical properties of cubic BC2N, a new super-hard phase), respectively. This class of materials exhibit outstanding mechanical performance such as ultra- high hardness, very low compressibility, toughness, and wear resistance, which can be utilized in widespread applications, such as cutting and polishing tools in machine industry, drilling bits in milling, mining and petrochemical industry, and diamond anvil cells in high pressure material science.
On account of its highly symmetric sp3 hybridized crystal structure, and presence of strong covalent C–C bonds, diamond possesses a unique combination of physical properties. Its hardness and thermal conductivity are the highest among covalent materials. These properties are extremely important for a wide range of applications in both industrial and scientific areas. Despite these outstanding advantages, however, diamond is extremely brittle, associated with inferior toughness and poor deformability. These shortcomings have caused undesirable tool chipping and breakage and have imposed severe constraints on technological innovations. The well-known trade-off between hardness and toughness (resistance to fracture) makes concurrent improvement of both properties a challenging endeavour, especially in diamond.
Increasing its hardness stillfurther (for instance, to achieve better machining efficiency and precision,or higher achievable pressure ranges in diamond anvil cells) usually results in lowering its toughness, and thereby,reducing the lifetime of diamond tools.Improving toughness and hardness simultaneously is a challenging taskbecause of the intrinsic brittleness of diamond. Moreover, poor thermal stability has also limited its applications, especially at high temperatures.The oxidation of diamond occurs at approximately 700 °C in air, which results in catastrophic loss of its mechanical properties and limits its widespread applications under oxidizing conditions. Currently, diamond can cut only non-ferrous materials and rocks, corresponding to a volume of 75%-80% of the total tool machining market.
Cubic boron nitride (cBN) is the second-hardest commercially used super hard material after diamond. cBN has a cubic crystal structure analogous to that of diamond, with each boron atom bonded with four nitrogen atoms and each nitrogen atom bonded with four boron atoms in a sp3 configuration. The main advantage of cBN over diamond is that it can be used to machine ferrous materials.While diamond reacts with ferrous metals, c-BN can maintain its chemically inert nature up to 1500-1600 K. CBN tools have more chemical and thermal stability over diamond tools (Recent Advances in SuperhardMaterials).
Polycrystalline cubic boron nitride (PCBN) exhibits excellent cutting tool properties and used to machine cast iron, heat-resistant alloys, and iron with high hardness (HRC value 45 and above), for example, shaft, bearings, engine parts, engine block, gears, and automobile parts. It is also suitable in the form of abrasive for high-speed grinding applications. The PCBN tools are used to machine superalloys with good dimensional accuracy and surface finish under dry conditions at high cutting speed. The partial ionicity of covalent B–N bonds in cBN lowers its intrinsic hardness. The hardness of synthetic cBN single crystal is only half that of diamond single crystal. Meanwhile,the fracture toughness of cBN single crystal is also about half that of diamond single crystal due to its exposure to the harsh synthesis process.
Currently, worldwide intense research efforts are being directed towards achieving very high toughness of super hard/ultra-hard materials through microstructural engineering while retaining their high hardness and thermal stability with the goal of achieving materials harder than natural diamond. In this connection, to design and develop nanotechnology-enabled super hard/ultra-hard materials is one of the most suitable strategies available to attain this goal. Nanocrystalline/ nano-twinned microstructures have been developed using innovative processing routes, which display significantly higher toughness, hardness, strength and thermal stability than that of traditional single crystal or polycrystalline diamond or cBN.
In one of the studies (Ultrahard stitching of nanotwinned diamond and cubic boron nitride in C2-BN composite), researchers from USA, China and Germany have Synthesized Cx-BN nanocomposites by reacting diamond nano-powder with BN atmoderate pressures and high temperatures.The nanocomposites consisted of randomly-oriented diamond and cBN domains stitched together by sp3-hybridized C-B and C-N bonds, leading to p-type semi-conductivity. Dislocations near the sutures accommodate lattice mismatch between diamond and cBN. Nano- twinning within both diamond and cBN domains further contributes to a bulk hardness ~50% higher than sintered cBN. Depending on the composition, the measured indentation hardness value of Cx-BN ranged between 40-85 GPa. The nanocomposite of C2-BN exhibitsthe highest Vickers hardness of 85 GPa (among the Cx-BNnano-composites), p-type semiconductivity with low activation energy and high thermal stability(the onset temperature of oxidation Tox is 1183 K), making it a functional, ultrahard substance.
In order to overcome the inherent limitations of diamond (prone to oxidation even at moderate temperatures and not suitable for high-speed cutting and polishing of ferrous alloys) and cBN{(its hardness is much lower than that of diamond (47 GPa for cBNvs85 GPa for diamond)}, the synthesis of transparent bulk diamond-cBN alloy compacts was carried out and samples were investigated for their thermal stability, hardness and cutting characteristics(Diamond-cBN alloy: A universal cutting material). The testing results showed that the diamond-cBN alloy has superior chemical inertness over polycrystalline diamond and higher hardness than single crystal cBN. The onset oxidation temperature of the diamond-cBN alloy (1070 K) was established from the thermal gravimetric curves, which was higher than that of polycrystalline diamond (869 K). The Vickers hardness of a diamond-cBN alloy bulk sample prepared at 19 GPa/2300 K was ranging between 74-78 VHN depending on the applied load. These values far exceeded the hardness of single crystal cBN (Hv 47 GPa). High-speed cutting tests on hardened steel and granite suggest that diamond-cBN alloy is indeed a universal cutting material.
Natalia Dubrovinskaia et al. have synthesized aggregated super-hard cBN nanocomposites which showed improvement in hardness of up to 100% in comparison with single crystal cBN (Super-hard nanocomposite of dense polymorphs of boron nitride: Noncarbon material has reached diamond hardness). The decrease of the grain size down to 14 nm and the simultaneous formation of the two different dense BN phases having hexagonal and cubic structures within the grains at nano(40nm)- and sub-nano levels (14nm) was attributed to its enormous mechanical property enhancement with maximum hardness of 85 GPa. This novel material also has an unusually high fracture toughness K1C = 15 MPa m0.5. in combination with high thermal stability above 1600 K in air, making it an exceptional super-abrasive.
Successful synthesis of single-phase nanocrystallinecBN was performed by Solozhenko et al. (Creation of Nanostuctures by Extreme Conditions: High-Pressure Synthesis of Ultrahard Nanocrystalline Cubic Boron Nitride)by direct solid-state phase transformation of graphite-like BN with “ideal random layer” (turbostratic) structure at 20 GPa and 1770 K. The material shows very high hardness (HV = 85 GPa), superior fracture toughness (KIc = 10.5 MPa·m1/2), and high thermal stability and oxidation resistance (up to 1500 K).
Yusheng Zhao et al. (Enhancement of fracture toughness in nanostructured diamond–SiC composites) synthesized diamond–SiC nanocomposites with super-hardness and greatly enhanced fracture toughness through a synthetic approach based on high-energy ball milling to form amorphous Si precursors followed by rapid reactive sintering at high pressure and high temperature. In their samples the measured fracture toughness KIC of the synthesized composites had been enhanced greatly, as much as 50% from 8.2 to 12.0 MPa m1/2, as the crystal size of the SiC matrix decreases from 10 mm to 20 nm. The fracture toughness KIC12 MPa m1/2 for the diamond–SiC composites is significant; it is about 30% tougher than tungsten carbide,which has the fracture toughness of 8.9 MPa m1/2 and is a hard and tough material often used for harsh applications where brittle polycrystalline diamond compacts would not work.
In recent past,a composite comprising nano-twin (nt) structured diamond co-existing with a new monoclinic crystalline form of diamond was prepared using a precursor of onion carbon (a high-energy meta stable carbon consisting of concentric graphite-like shells nanoparticles) (Nanotwinned diamond with unprecedented hardness and stability).The pure synthetic bulk nt-diamond material shows remarkablehardness (Vickers) of upto 200GPaand superior thermal stability, associated with an in-air oxidization temperature more than 200 0C greater than that of natural diamond. The fracture toughness values were also reasonable ranging from 9.7 to 14.8 MPa m 0.5.
In another study (Hierarchically structured diamond composite with exceptional toughness), a hierarchically structured diamond composite with unique architecture (interwoven nanotwins and interlocked nanograins)consisting of diamond nanotwin domains along with thin slices of meta stable carbon phases epitaxially confined within diamond nanotwins was preparedfrom onion carbon nanoparticlesat an optimum pressure > 18 GPa(Using a Diamond‐anvil pressure cell).It exhibits extreme fracture toughness of above 25 MPa m1/2 due to multiple toughening mechanisms and a Vickers hardness of 200 GPa.
All the above stated examples clearly highlightsthe importance of microstructure engineering in tailoring mechanical properties in super-hard and ultra-hard materials.
In metallic materials also a similar strategy of microstructural engineering has been adopted to achieve high strength associated with superior toughness ((For example: Nanocrystalline and nano-twinned microstructures; gradient nanostructures; nano-laminated structures including bimetal nanolaminate; heterogeneous harmonic structures; and heterogeneous lamellar structures,(Nanocrystalline Materials)). In a remarkable example (Heterogeneous lamella structure unites ultrafine-grain strength with coarse-grain ductility), this strategy has been implemented in titanium for the sake of evading strength–ductilitytrade-off by designing a heterogeneous lamellar structure, i.e., soft microscale-grained lamellae embedded in hard ultrafine-grained lamellarmatrix. The heterogeneous deformation of this newly found structure produces significant back-stress hardeningin addition to conventional dislocation strengthening, making it susceptible to higher strain hardening than coarse-grained metals. The high back-stress hardening makes the material as strong as ultrafine-grained metals and as ductile as coarse-grained metals., which is especially,problematic to achieve for ultra-strong ultrafine-grained metals.
In view of the above advances made in metallic materials, alternative microstructure engineering approaches other than nano-structuring and nano-twinning alone need to be explored in super-hard materials to further enhance their properties to a considerable extent.Unfortunately, in super-hard diamond and cBN,atoms are held together by strong covalent bonds with different arrangements unlike metallic materials which are amenable to plastic deformation. Therefore, processing techniques such as severe plastic deformation suitable for metals are not feasible in the case of super-hard materials. In view of this difficulty, alternative techniques like compositing approach may find solution to overcome this dilemma in super-hard materials.
In the present work, heterogeneously structured diamond−cBN composites composed of lamellar coarse grained cBN regions tightly embedded in ultrafine nano-twinned diamond (nt-diamond) zones were successfully synthesized from an intricately prepared precursor, i.e., hBN micro-flakeswrapped with Onion-like carbon(OLC) nanoparticles, under high pressure high temperature (HPHT) conditions. It is of interest to note that structurally, the composite displays a microstructure similar to that of heterogeneous lamellar structured titanium (Nanocrystalline Materials) i.e., elongated coarse grained cBN regions embedded in an ultrafine nt-diamond matrix. The diamond−cBN composite displays an appealing Knoop hardnessof 85 GPa and a fracture toughness as high as 16.9 MPam1/2that isappreciably tougher than both the diamond and cBN components.Concurrently, the composite also shows superb thermal stability in an atmospheric air oxidization temperature up to 1022 °C (1295 K).
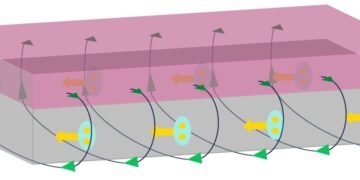
On account of this distinctive microstructure that has been created in strong covalent materials for the first time, multiple toughening mechanisms, such as nanotwin toughening, crack bridging, deflection, and branching, pull-out fracture, and frictional sliding are occurring. As conceptualized in Figure 1, this phenomenon can contribute synergistically to enhance the fracture toughness of the heterogeneous diamond−cBN composite.
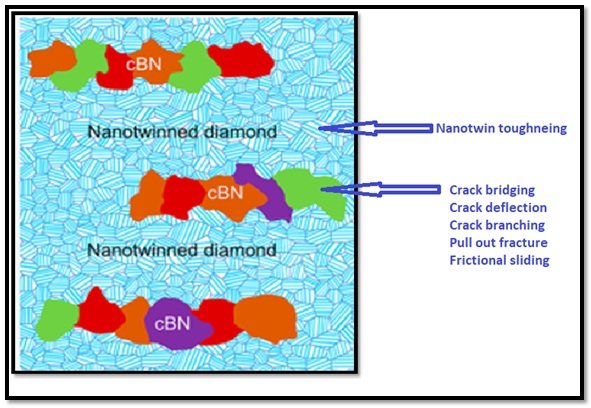
Figure 1) Artist view of Heterogeneous diamond-cBN composites showing lamellar coarse grained cBN regions embedded in ultrafine nano-twinned diamond (nt-diamond) zones (matrix). It also shows synergistic toughening mechanisms, including nanotwin toughening, crack bridging, deflection and branching of cracks followed by pull out fracture and frictional sliding of fractured grains.
Reprinted and adapted with permission from (Heterogeneous Diamond-cBN Composites with Superb Toughness and Hardness, Baozhong Li, Pan Ying, YufeiGao, et al., Nano Letters, American Chemical Society, Jun 1, 2022) Copyright © 2022, American Chemical Society
In this study, precursors of hBNmicroscale flakes uniformly wrapped up by OLC nanoparticles(just the way bricks are encased with mortar) were prepared by an electrostatic self-assembly method (Electrostatic Self-Assembly: Understanding the Significance of the Solvent).
The diamond−cBN composite was synthesized at 15 GPa and 2000 °C from the aforementioned precursor comprising hBNmicroscale flakes and OLC nanoparticles (with a mass ratio of 1:1). The samples of diamond-cBN composites were characterized using X-ray Diffraction(XRD) and high-resolution scanning transmission electron microscopy (STEM). It was observed that under HPHT, hBN micro-flakes individually wrapped with OLC nanoparticles are transformed into micron-sized cBN regions intimately embedded in the nanostructured diamondmatrix, forming a heterogeneous microstructure with a duplex grain size distribution: i.e., ultrafine grains (ca. 300 nm average) in cBN regions and nanograins (less than 50 nm average) in diamond regions.The diamond nanograins showed the presence of a high density of lamellar {111} nanotwins. XRD characterization studies of the as obtained samples showed only those peaks corresponding to diamond and cBN and there was total absence of any other peaks suggesting that a complete transformation of precursor has occurred.
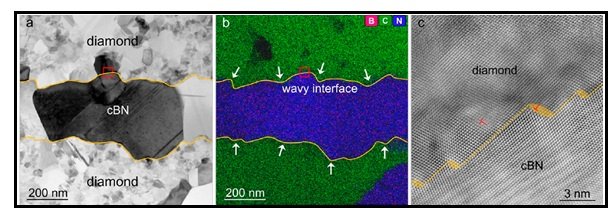
Figure 2) Characterization of the heterogeneous interface between diamond and cBN. (a, b) Representative bright field BF-STEM image and the corresponding EDS mapping of diamond and cBN regions in the composite, revealing a clear and wavy interface between. (c) HAADF-STEM image from the red-boxed area in (a) and (b) showing an atomic-scale steplike interface between diamond and cBN accompanied by dislocations, taken along the [101̅] zone axis of diamond and cBN.
Reprinted and adapted with permission from (Heterogeneous Diamond-cBN Composites with Superb Toughness and Hardness, Baozhong Li, Pan Ying, YufeiGao, et al., Nano Letters, American Chemical Society, Jun 1, 2022) Copyright © 2022, American Chemical Society.
As depicted in Figure 2 (a,b), TEM characterization studies ((BF-STEM and Energy-dispersive X-ray spectroscopy (EDS)) have revealed the presence of clear intimately bound wavy interfaces between the diamond and cBN regions. Occasionally, straight interfaces are also observed in the composite (not shown in this Figure). TheHAADF-STEM image shown in Figure 2c reveals an atomic scale hetero interface between the diamond and cBN with coincident [101̅] zone axes. Evidently, the step like features offsetting the diamond−cBN interface (yellow-shadowed areas) and misfitdislocations can effectively accommodate the strains that exist (induced by the lattice mismatch of about 1.3% between cBN and diamond interfaces)near theinterfaces, leading to the formation of an epitaxial heterointerfacebetween diamond and cBN.
Structurally, the composite possesses a microstructure that is akin to the heterogeneous lamellar structured titanium (Heterogeneous lamella structure unites ultrafine-grain strength with coarse-grain ductility)(i.e., elongated coarsegrained cBN regions entrenched firmly into an ultrafine nt-diamondmatrix). Due to this distinctive microstructure that has beenachieved in strong covalentlybonded materials for the first time, multiple toughening mechanisms, such as nanotwin toughening and crack bridging in nt-diamond region; crack deflection and crack branching while the crack propagating from diamond region into cBN region;pull-out and fractional sliding between diamond and cBN grains, all these events can contribute synergistically to energy dissipation and, in turn, enhancing thefracture toughness of the heterogeneous diamond−cBN composite.
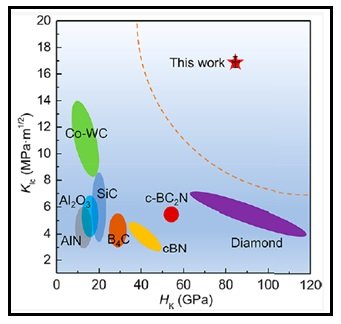
Figure 3) Shows the fracture toughness (KIC) as a function ofKnoop hardness (HK) of the diamond−cBN composite and for the sake of comparison, the plot also includes variousother competing ultra-hard and super-hard materials.
Reprinted and adapted with permission from (Heterogeneous Diamond-cBN Composites with Superb Toughness and Hardness, Baozhong Li, Pan Ying, YufeiGao, et al., Nano Letters, American Chemical Society, Jun 1, 2022) Copyright © 2022, American Chemical Society.
In Figure 3, the fracture toughness is plotted as a function of Knoop hardness for thent-diamond-cBN composite as well as other competitive ultra-hard/super-hard materials, including single crystal diamond (KIC =about 5 MPa m1/2), single crystal cBN(KIC =2.8 MPa m1/2), c-BC2N (KIC =4.5 MPa m1/2) and traditional cutting tool material, Co-WC cemented carbide (KIC= approx. 12 MPa m1/2). The nt-diamond-cBN composite exhibits outstanding combination of fracture toughness (KIC =16.9 ± 0.8 MPa m1/2) and Knoop hardness (HK – 85 GPa). It outperforms all other competing super-hard/ultra-hard materials as far as the fracture toughness is concerned while retaining its reasonably high hardness of 85 GPa. These mechanical properties are ideally suited for cutting tool materials for high performance applications. Another major requirement for the high-speed machining operation is the thermal stability of the cutting tool. Nt-diamond-cBN composite would certainly meet this requirement because its onset oxidization temperature of 1022 °C is substantially higher than those of diamond crystals (595.85 °C) and homogeneous diamond−cBN alloys(796.85 °C)(Diamond-cBN alloy: A universal cutting material).
This work provides a novel strategy to design and develop heterogeneously structuredultra-hard/super-hard materials having outstanding fracture toughness while retaining their high hardness. These high-performance materials will find applications in major industries like machining, electronics, construction and petrochemicals, among others.
About Author

Dr Yashwant R Mahajan was Technical Advisor at International Advanced Research Centre for Powder Metallurgy and New Materials (ARCI), Hyderabad, and also at ARC-I’s Centre for Knowledge Management of Nanoscience and Technology (CKMNT), Hyderabad. He obtained his Ph.D. degree in Physical Metallurgy in 1978 from Polytechnic Institute of Brooklyn, New York. Dr Mahajan made major contributions in the areas of MMCs, advanced ceramics, and CMCs. Under his leadership, a number of ceramic- based technologies were developed and transferred to the industry. He has published more than 130+ technical papers in peer-reviewed journals and conference proceedings and holds 13 patents (including 2 US). Dr Mahajan is a recipient of NRC Associateship of National Academy of Sciences, USA, the best metallurgist of the year award (IIM), MRSI Medal, MRSI-IISC superconductivity award, and VASVIK Medal. Dr Yashwant R Mahajan is also the Editorial Adviser to Nano Digest. Email: mahajan@nanodigest.in