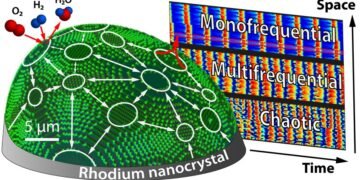
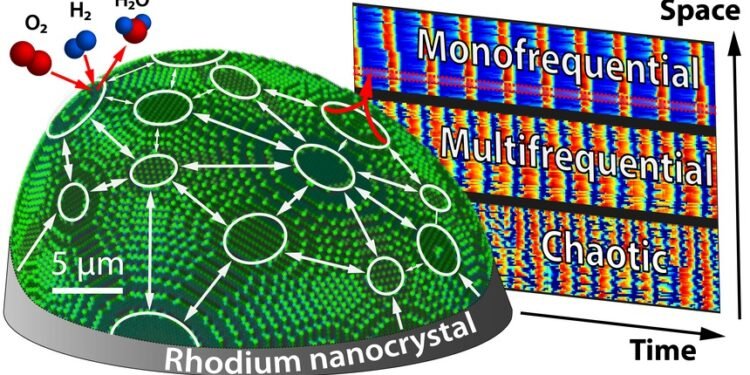
Sometimes a chemical reaction not only proceeds in one direction, but exhibits spatio-temporal oscillations. At TU Wien, changes in chaotic behavior (Nanoscale Chaos) are observed at the nanometer scale.
Turbulent behavior is often characterized by large systems: for example, the atmosphere, asteroids and the universe that are attracted by many large bodies at the same time, or a pendulum that swings together. However, in the atomic scale, one does not encounter chaos – other effects usually occur. Now, for the first time, scientists at the TU Wien have been able to detect clear indications of chaos at the nanoscale – in chemical reactions in small crystals of rhodium. The results were published in the journal Nature Communications.
From inactive to active – and vice versa
The chemical reaction born is really simple: using precious metals, oxygen uses hydrogen to regenerate water, which is the basic principle of fuel cells. The reaction rate depends on the external conditions (pressure, temperature). Under certain conditions, however, this reaction exhibits an oscillating behavior, even if the external conditions are constant. “In the same way that a pendulum swings from left to right and back, the rate of reaction moves between the incomprehensible and the sublime, therefore, a system of stimuli that moves between inactive and active states,” explains the professor. Günther Rupprechter from the Institute for Materials Chemistry at TU Wien.
A pendulum is a beautiful example of something that can be seen – if you bend it a little or make it swing twice in a slightly different direction, it does the same thing. In this sense, it is the opposite of a chaotic system, where minute conflicts in the initial situation lead to different results and long-term behavior. An excellent example of this practice is a number of pendulums attached to a rubber band.
Specifying exactly the same initial condition twice is impossible
“In principle, of course, the laws of nature always directly determine the behavior of pendulums”, says Professor Yuri Suchorski (TU Wien). “If we can start such a system in which the pendulums are connected in the same direction twice, the pendulums will move in the same direction twice.” But in practice, that’s impossible: you can’t reproduce the same first situation as the second one – and even a small difference in the first situation will make the system behave differently than the first. famous “butterfly effect”: a small difference in the first situation leads to a big conflict in the state at a later time.
A similar phenomenon is now observed during chemical oscillations in the rhodium nanocrystal: “the crystal is composed of many different surface nanofacets, like a polished diamond, but smaller, in order of nanometers”, explained Maximilian Raab and Johannes Zeininger, who conducted the experiment. “In each of these parts, the chemical reactions are repeated, but the reactions in the neighboring parts are connected.”
Change – from order to chaos
The current bonding behavior can be controlled in a significant way – by changing the amount of hydrogen. At first, one side controls and sets the tone as the pacer. All the other parts come together and vibrate to the same tune. If the hydrogen concentration increases, the situation becomes more complicated. Different parts oscillate in different ways – but their behavior is always regular and efficient. However, if the hydrogen concentration is increased, this process suddenly fails. Chaos prevails, oscillations become unpredictable, small differences in the initial conditions lead to completely different oscillations – a clear sign of chaos.
“This is significant because one would not expect chaotic behavior in nanoscale structures,” says Yuri Suchorski. “The smaller the system, the greater the contribution of stochastic noise. In fact, noise, which is very different from chaos, should control the behavior of the system: it is more interesting that it will possible to “remove” the manifestation of chaos. A particularly useful program model, developed by Professor Keita Tokuda (University of Tsukuba).
Chaos analysis is applied to nanochemistry
Günther Rupprechter says, “The study of chaos theory has been going on for decades and has already been focused on chemical reactions in large (macroscopic) systems, but our study is the first attempt to convey a deeper understanding from this area at the nanoscale,” says Günther Rupprechter. . “Small distortions in crystal symmetry can determine whether an object behaves in an orderly and predictable manner or in an erratic and chaotic manner. This is important for different chemical reactions – and possibly even for the course of life”.
Source: TU Wien