Solid-state batteries for electric vehicles, offering greater energy density and range than contemporary lithium-ion batteries, remain out of reach, not least due to challenges coming from the composition of the battery’s cathode. Re-jigged cathode recipe gives new hope to solid-state batteries for electric vehicles. A new cathode composition and accompanying manufacturing technique looks set to overcome this hurdle for Solid state batteries for electric vehicles of lithium-ion batteries.
A paper describing the manufacturing process appeared in the journal Nano Research on Mar. 24.
Rechargeable solid-state batteries (ones that are completely solid, with no liquid components) have long been sought as the next generation of energy storage, not least for electric vehicles and other climate mitigation applications. They would be lighter, more energy dense, offering greater range and faster recharging than the current generation of lithium-ion batteries.
The liquid electrolyte used in the latter is the medium through which current flows between the positive and negative electrodes (the cathode and anode, respectively). But the liquid makes the battery heavy. It’s also flammable and fires are not an uncommon occurrence. In a solid-state battery, a solid electrolyte made of ceramic, glass or a polymer is much safer as there are no leaks or splashing about while in transit, and offers improved power density, cyclability and shelf life.
Key to making solid-state batteries work is designing a good cathode that it is capable of a high operating voltage and high area capacity. The latter term describes the amount of energy charge in a battery per unit of area for a given period of time. The unit commonly used to describe this quantity is the milliampere-hour (mAh)—or the amount of energy charge that will allow one amp of current to flow for an hour—compared to a given amount of area (typically measured in square centimeters, or cm2). In essence, this measurement, mAh/cm2, offers an indication of how long a battery will last without having to recharge it, for the amount of space it takes up in a device.
“Most of the composite cathode manufacturing technologies that have been explored so far result in batteries that do not even match the performance of existing commercial batteries, let alone exceed them, hitting around 3 mAh/cm2,” said Jizhang Chen of the College of Materials Science and Engineering at Nanjing Forestry University and lead author of the paper.
These cathode technologies also suffer from the need for the addition of an excessive amount of binders and conductive agents to ensure that all the active particles are uniformly spread out. This reduces the density of the cathode, increases the cost, and also produces a great deal of resistance at the interface of the cathode and electrode.
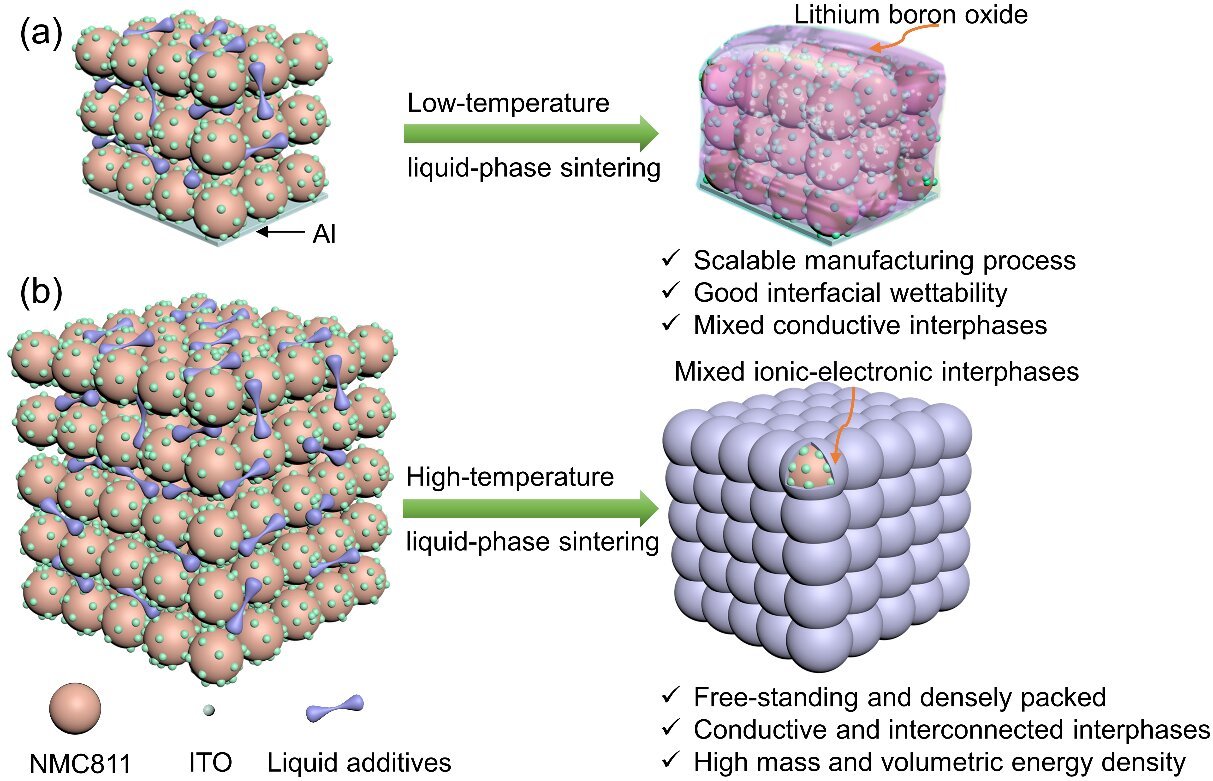
So the researchers developed a novel cathode composition and accompanying manufacturing technique that overcomes these challenges while offering a high area capacity. The quantity of binders and conductive agents, in this case lithium hydroxide and boric acid, added is substantially reduced (down to about four percent of the overall weight). These are used as additives in the sintering process during cathode formation.
Sintering is a method of compacting a powder into a solid mass via heat or pressure without melting it to the point of becoming a liquid. In this case however, there remains a liquid phase for at least some components while others remain powder in order to give a boost to the bonding between particles.
The lithium hydroxide and boric acid, with their low melting points, infiltrate as liquids into a powder of a nickel-rich lithium compound (LiNi0.8Mn0.1Co0.1, or “NMC811”) at a moderately elevated temperature (around 350℃). This not only enables intimate physical contact between the powder particles, it also reduces the need for a high quantity of additives and promotes a densification process.
The resulting composite cathode delivered promising performance, hitting an area capacity above 8 mAh/cm2 within a wide range of voltages up to 4.4 V. This is expected to be used to manufacture solid-state batteries with an energy density of 500 watt-hours per kilogram (Wh/kg), easily beating the 100-265 Wh/kg energy density offered by contemporary lithium ion batteries.