“Identifying new bacterial targets and alternative strategies (New model for antibacterial mechanism) to control bacterial growth will be even more important,” said Brookhaven biologist Paul Freimuth, who led the study. Bacteria will develop resistance to many commonly used drugs, and many scientists and doctors are concerned about the potential for large-scale attacks caused by these antibiotic-resistant bacteria, he explained. “What we’ve discovered is far from curing, but the first step is to understand the mechanism,” Freimuth said. “We know of egg protein, which mimics the effect of a complex mixture of aberrant egg proteins produced when bacteria are treated with aminoglycosides. It gives us a way to study the mechanism that kills bacterial cells. Then a new family of regulators can be developed to do the same.”
After an interesting branch
Brookhaven researchers, who often focus on energy-related research, did not think about human health when launching the project. They use E. coli to study genes involved in the construction of plant cell walls. This research (New model for antibacterial mechanism) will help scientists learn how to more efficiently convert plant material (biomass) into biofuels.
But when it triggers the expression of a particular plant gene and allows the bacteria to make egg white, the cells immediately stop growing.
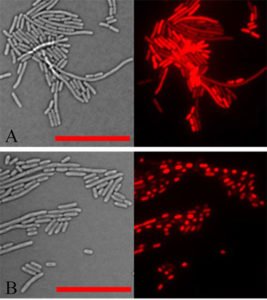
“This protein has a serious toxic effect on cells. All cells die within minutes of learning to express this gene,” Freimuth said. Understanding the basis for this rapid inhibition of cell growth makes it an ideal research project for summer interns working in Freimuth’s laboratory.
“Trainees can conduct experiments and see the effects one day,” he said. And maybe they can help figure out why a vegetable protein can cause serious damage.
Bad code reading, unwrapped egg whites
“Then it started to get interesting,” Freimuth said.
The group for New model for antibacterial mechanism found that plant protein was not a toxic factor. It was a poison of amino acids, the building blocks of egg whites, pointless.
This useless chain is accidentally broken when bacterial ribosomes (cells that make up a protein machine) translate the letters that make up the genetic code “out of phase.” Instead of reading the code of the three-letter fragments that encode a particular amino acid, the ribosome reads only the second two letters of the fragment and the first letter of the next triplet. This results in the placement of incorrect amino acids.
“It’s like reading a sentence that starts in the middle of each word and associating it with the first half of the next word, creating a lot of nonsense,” Freimuth said.
Babble protein is reminiscent of Freimuth’s class of antibiotics called aminoglycosides. These antibiotics force ribosomes to make similar “phasing” errors and other types of errors in egg protein production. The result: all bacterial ribosomes produce unnecessary egg whites.
“If a bacterial cell has 50,000 ribosomes, each producing a different aberrant protein, is the toxic effect the result of a specific aberrant protein or a combination of many? This issue arose decades ago and still needs to be addressed, Said Freimuth.
New research (New model for antibacterial mechanism) shows that only one aberrant protein may be sufficient for a toxic effect. That’s not too far. Useless amino acid chains may not compose well enough to be fully functional. Although incorrect doubling of egg whites in all cells can be caused by accidental errors, it is often detected and completely eliminated by a machine for “quality control” of healthy cells. Disruption of quality control systems can cause the accumulation of abnormal proteins and cause disease.
Damaged quality control
The next step is to find out if the abnormal plant protein can activate a bacterial cell quality control system – or somehow prevent that system from working. Freimuth and his team found that the aberrant plant protein actually activated the first step of protein quality control, but the later stages of the process, which are immediately necessary for the degradation of the aberrant proteins, are blocked. They also found that the difference between cell life and death depends on the rate of abnormal protein production.
“When cells have multiple copies of a gene that encodes an aberrant plant protein, the quality control machine detects the protein but can’t completely degrade it,” Freimuth said. “However, when we reduced the copy number of the gene, the quality control machine was able to eliminate the toxic protein and the cells were preserved.”
The same is said, he said, in cells treated with sublethal doses of aminoglycoside antibiotics. “The quality control response has been activated quickly, but the cells are still growing,” he said. Model for mechanism
These experiments show that aberrant plant protein kills cells by the same mechanism with complex mixing of aberrant egg proteins caused by aminoglycoside antibiotics. But the exact mechanism of the same death is still a mystery.
“The good news is that we now have a protein with a known amino acid sequence that we can use as a model to test this mechanism,” Freimuth said.
Researchers know that cells treated with antibiotics can leak, allowing things like salts to absorb up to toxic levels. One hypothesis is that improperly propagated egg proteins may form new channels in cell membranes or alternatively open the gates of existing channels, allowing the spread of salts and other toxic substances in the cell membrane. “The next step is to determine the structures of our protein in complex with membrane channels to investigate how the protein can inhibit normal channel function,” Freimuth said.
This would help improve understanding of how aberrant proteins caused by aminoglycoside antibiotics can kill bacterial cells – and may be the basis for designing new drugs that produce similar or similar effects.