Researchers from the Institute of Industrial Science at The University of Tokyo used molecular dynamics calculations to simulate the glass-forming ability of metallic mixtures. They show that even small changes in composition can strongly influence the likelihood that a material will assume a crystalline versus a glassy state upon cooling. This work may lead to a universal theory of glass formation and cheaper, more resilient, electroconductive glass.
If you have important guests coming over for dinner, you might set your table with expensive “crystal” glasses. To scientists, however, crystal and glass are actually two very different states that a liquid might assume when cooled. A crystal has a defined three-dimensional lattice structure that repeats indefinitely, while glass is an amorphous solid that lacks long-range ordering. Current theories of glass formation cannot accurately predict which metallic mixtures will “vitrify” to form a glass and which will crystallize. A better, more comprehensive understanding of glass formation would be a great help when designing new recipes for mechanically tough, electrically conductive materials.
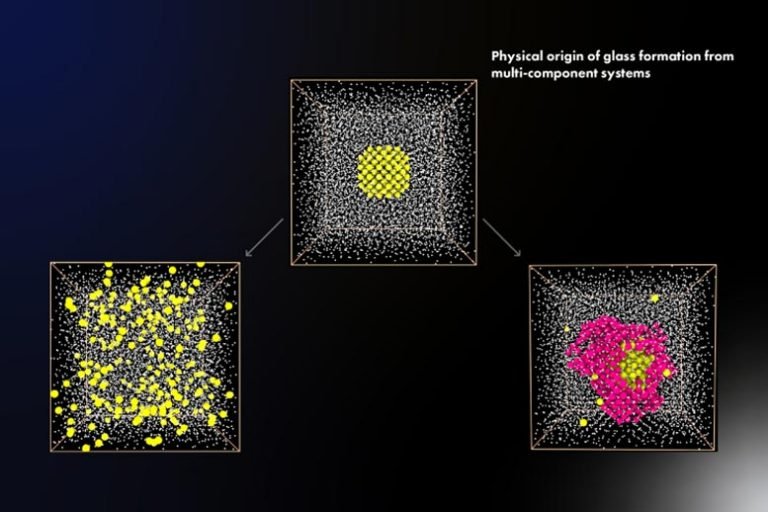
Now, researchers at the University of Tokyo have used computer simulations of three prototypical metallic systems to study the process of glass formation. “We found that the ability for a multi-component system to form a crystal, as opposed to a glass, can be disrupted by slight modifications to the composition,” first author Yuan-Chao Hu says.
Stated simply, glass formation is the consequence of a material avoiding crystallization when cooled. This locks the atoms into a “frozen” state before they can organize themselves into their energy-minimizing pattern. The simulations showed that a critical factor determining the rate of crystallization was the liquid-crystal interface energy.
The researchers also found that changes in elemental composition can lead to local atomic orderings that frustrate the process of crystallization with arrangements incompatible with the crystal’s usual form. Specifically, these structures can prevent tiny crystals from acting as “seeds” that nucleate the growth of ordered regions in the sample. In contrast with previous explanations, the scientists determined that the chemical potential difference between the liquid and crystal phases has only a small effect on glass formation.
“This work represents a significant advancement in our understanding of the fundamental physical mechanism of vitrification,” senior author Hajime Tanaka says. “The results of this project may also help glass manufacturers design new multi-component systems that have certain desired properties, such as resilience, toughness and electroconductivity.”
Source: University of Tokyo.