Scientists from the University of Leipzig, together with colleagues from the University of Vilnius in Lithuania, have developed a new method to measure (Researchers Observe the Procedure of CRISPR Genetic Sissors) the small complexity and strength of cells in milliseconds. This method analyzes the gene recognition of the CRISPR-Cas protein complex, also known as “gene recognition“, in real time and with the highest resolution.
From the data obtained, the identification process will be used to better understand and improve the importance of genetics. The results of the team led by Professors Ralf Seidel and Dominik Kauert from the Department of Physics and Earth Sciences have been published in the famous journal Nature Structural and Molecular Biology.
When bacteria are attacked by viruses, they can defend themselves with mechanisms that block the genes introduced by the intruder. Key features of the CRISPR-Cas protein complex, It is only in the last decade that their anti-mutagenic role in microorganisms has been discovered and described.
Using complementary RNA, the CRISPR complex recognizes short sequences in the invader’s DNA. RNA sequence recognition techniques are used to selectively turn off and modify genes in each organism. This discovery has revolutionized genetic engineering and has already been rewarded in 2020 with the Nobel Prize in Chemistry awarded to Emmanuelle Charpentier and Jennifer A. Dudna.
However, sometimes, the CRISPR company reacts to a part of the gene that is different from the sequence of the said RNA. This leads to unwanted effects on medical devices. “The reason for this is not well known, because the process could not be seen directly until now,” says Dominik Kauert, who worked on the project as a doctoral student.
The NANOSCALE method follows closely
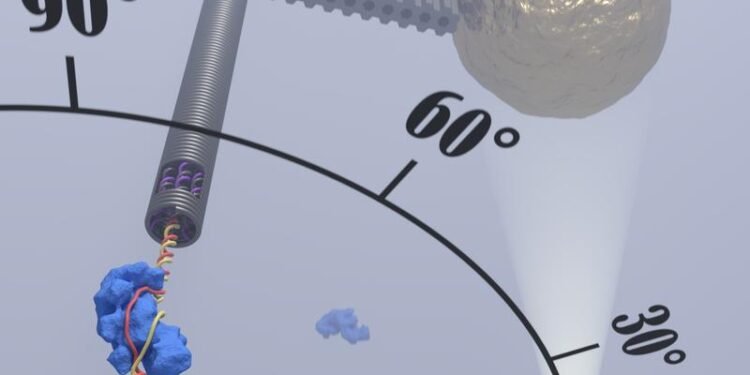
To better understand the recognition process, the team led by Professors Ralf Seidel and Dominik Kauert took advantage of the fact that the DNA double helix of the target sequence is not damaged during the recognition process to allow the binding of the core to the target sequence. Kauert explains: “Thus, the main question in the work is whether the unfolding of a single piece of DNA only 10 nanometers (nm) in length can be followed in real time.
“To see the process of exposure properly, scientists must visualize it under a microscope. To achieve this goal, the group relies on the achievement of DNA nanotechnology, which can be used to make any three DNA nanostructures. Using a technique called DNA origami, the researchers produced a 75 nm long DNA rotor arm with a gold nanoparticle attached to its end. In the experiment, a DNA sequence of 2 nm in length and 10 nm in length is moved in the rotation of a gold nanoparticle along a circle with a diameter of 160 nm – this movement can be magnified and followed using a microscope unique improve.
With this new method, the researchers were able to see the sequence recognition of the CRISPR Cascade complex which is almost two-fold from the main path. Surprisingly, base binding to RNA is very poor, which means that the complex is bound only during sequence recognition. It is only when all sequences are identified that a stable bond emerges and breaks down the DNA. If it is an “invalid” target sequence, the sequence is aborted.
The results can help choose the right RNA sequence
The fact that the cream system sometimes produces wrong results is due to its stochastic nature, the translation of the mysterious movement, as researchers have been able to show now. Kauert explains, “the process sensitivity is the temperature change in the base connection.” From the obtained data, it is possible to develop a thermodynamic characteristic system that describes the characteristics of the process components.
In the future, this should allow a better selection of RNA sequences that recognize only the desired target sequence, thus optimizing gene expression.
As the nanorotors engineers are universal in their ability to measure complexity and torques in single molecules, they can also be used for other CRISPR-Cas complexes or biomolecules.
The work was supported by the European Research Council and the German Research Foundation in collaboration with the research group of Professor Virginijus Siksnys of the University of Vilnius in Lithuania, which was isolated and provided to the CRISPR company.
Source: University of Leipzig