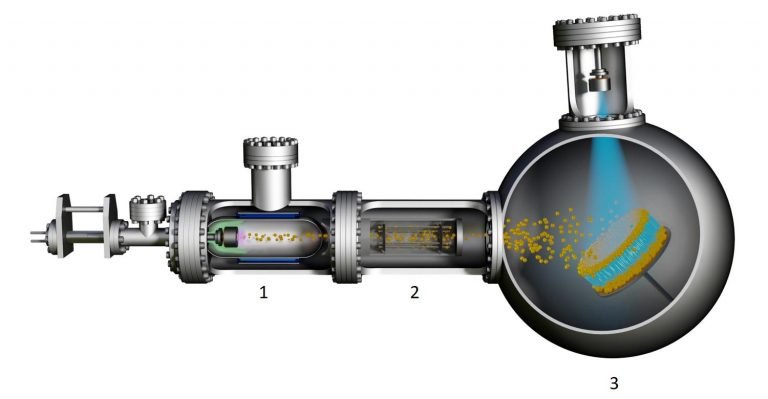
In chamber 1, the nanoparticles, made from tantalum metal, are grown. Within this chamber, individual tantalum atoms clump together, similar to the formation of rain droplets. In chamber 2, the nanoparticles are mass filtered, removing ones that are too large or too small. In chamber 3, a layer of nanoparticles is deposited. This layer is then “sprayed” with isolated silicon atoms, forming a silicon layer. This process can then be repeated to create a multi-layered structure. Credit: Schematic created by Pavel Puchenkov, OIST Scientific Computing & Data Analysis Section
Scientists reveal a new nanostructure that could revolutionize technology in batteries and beyond.
New research has identified a nanostructure that improves the anode in lithium-ion batteries
Instead of using graphite for the anode, the researchers turned to silicon: a material that stores more charge but is susceptible to fracturing
The team made the silicon anode by depositing silicon atoms on top of metallic nanoparticles
The resulting nanostructure formed arches, increasing the strength and structural integrity of the anode
Electrochemical tests showed the lithium-ion batteries with the improved silicon anodes had a higher charge capacity and longer lifespan
New research conducted by the Okinawa Institute of Science and Technology Graduate University (OIST) has identified a specific building block that improves the anode in lithium-ion batteries. The unique properties of the structure, which was built using nanoparticle technology, are revealed and explained today (February 5, 2021) in Communications Materials.
Powerful, portable and rechargeable, lithium-ion batteries are crucial components of modern technology, found in smartphones, laptops and electric vehicles. In 2019, their potential to revolutionize how we store and consume power in the future, as we move away from fossil fuels, was notably recognized, with the Nobel Prize co-awarded to new OIST Board of Governors member, Dr. Akira Yoshino, for his work developing the lithium-ion battery.
Traditionally, graphite is used for the anode of a lithium-ion battery, but this carbon material has major limitations.
“When a battery is being charged, lithium ions are forced to move from one side of the battery — the cathode — through an electrolyte solution to the other side of the battery — the anode. Then, when a battery is being used, the lithium ions move back into the cathode and an electric current is released from the battery,” explained Dr. Marta Haro, a former researcher at OIST and first author of the study. “But in graphite anodes, six atoms of carbon are needed to store one lithium ion, so the energy density of these batteries is low.”
With science and industry currently exploring the use of lithium-ion batteries to power electric vehicles and aerospace craft, improving energy density is critical. Researchers are now searching for new materials that can increase the number of lithium ions stored in the anode.
One of the most promising candidates is silicon, which can bind four lithium ions for every one silicon atom.

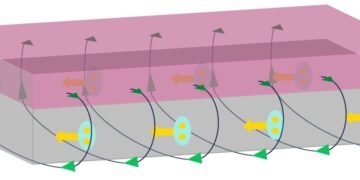
In the first stage, the silicon film exists as a rigid but wobbly columnar structure. In the second stage, the columns touch at the top, forming a vaulted structure, which is strong due to arch action. In the third stage, further deposition of silicon atoms results in a sponge-like structure. The red dashed lines show how the silicon deforms as a force is applied. Credit: Schematic created by Dr. Panagiotis Grammatikopoulos, OIST Nanoparticles by Design Unit and Particle Technology Laboratory, ETH Zürich
“Silicon anodes can store ten times as much charge in a given volume than graphite anodes — a whole order of magnitude higher in terms of energy density,” said Dr. Haro. “The problem is, as the lithium ions move into the anode, the volume change is huge, up to around 400%, which causes the electrode to fracture and break.”
The large volume change also prevents stable formation of a protective layer that lies between the electrolyte and the anode. Every time the battery is charged, this layer therefore must continually reform, using up the limited supply of lithium ions and reducing the lifespan and rechargeability of the battery.
“Our goal was to try and create a more robust anode capable of resisting these stresses, that can absorb as much lithium as possible and ensure as many charge cycles as possible before deteriorating,” said Dr. Grammatikopoulos, senior author of the paper. “And the approach we took was to build a structure using nanoparticles.”
In a previous paper, published in 2017 in Advanced Science, the now-disbanded OIST Nanoparticles by Design Unit developed a cake-like layered structure, where each layer of silicon was sandwiched between tantalum metal nanoparticles. This improved the structural integrity of the silicon anode, preventing over-swelling.
While experimenting with different thicknesses of the silicon layer to see how it affected the material’s elastic properties, the researchers noticed something strange.
“There was a point at a specific thickness of the silicon layer where the elastic properties of the structure completely changed,” said Theo Bouloumis, a current PhD student at OIST who was conducting this experiment. “The material became gradually stiffer, but then quickly decreased in stiffness when the thickness of the silicon layer was further increased. We had some ideas, but at the time, we didn’t know the fundamental reason behind why this change occurred.”
Now, this new paper finally provides an explanation for the sudden spike in stiffness at one critical thickness.
Through microscopy techniques and computer simulations at the atomic level, the researchers showed that as the silicon atoms are deposited onto the layer of nanoparticles, they don’t form an even and uniform film. Instead, they form columns in the shape of inverted cones, growing wider and wider as more silicon atoms are deposited. Eventually, the individual silicon columns touch each other, forming a vaulted structure.
“The vaulted structure is strong, just like an arch is strong in civil engineering,” said Dr. Grammatikopoulos. “The same concept applies, just on a nanoscale.”
Importantly, the increased strength of the structure also coincided with enhanced battery performance. When the scientists carried out electrochemical tests, they found that the lithium-ion battery had an increased charge capacity. The protective layer was also more stable, meaning the battery could withstand more charge cycles.
These improvements are only seen at the precise moment that the columns touch. Before this moment occurs, the individual pillars are wobbly and so cannot provide structural integrity to the anode. And if silicon deposition continues after the columns touch, it creates a porous film with many voids, resulting in a weak, sponge-like behavior.
This reveal of the vaulted structure and how it gains its unique properties not only acts as an important step forward towards the commercialization of silicon anodes in lithium-ion batteries, but also has many other potential applications within material sciences.
“The vaulted structure could be used when materials are needed that are strong and able to withstand various stresses, such as for bio-implants or for storing hydrogen,” said Dr. Grammatikopoulos. “The exact type of material you need — stronger or softer, more flexible or less flexible — can be precisely made, simply by changing the thickness of the layer. That’s the beauty of nanostructures.”