Supercapacitors are energy storage devices that complement rechargeable batteries, and can replace them in one part. The current bosses don’t have enough power, so they don’t last long. A new way to make a supercapacitor with a “breathing” electrode is very advanced. As the team that developed it explains in the journal Angewandte Chemie (“High Power- and Energy-Density Supercapacitors through the Chlorine Respiration Mechanism“), inspired lizards generate air bubbles when they breathe you swim underwater.
Today, applications for carriers include intermittent power outages in facilities such as hospitals or data centers, as well as over consumption from electronic devices. Load carriers charged by brake power enable modern trams and buses to run on electricity. They are also increasingly interested in solar power plants to stabilize voltage fluctuations.
Unlike rechargeable batteries, supercapacitors are “runners” of energy storage: they can generate a high amount of current in a short time (high energy density). However, they are poor “distance runners” because, even with low electricity, they do not last long (low power density). Modern electric storage will combine the two qualities and have a small weight. Unfortunately, the way to increase the current energy density always comes at the cost of energy density – a stumbling block for the development of supercapacitors.
A team of Long Chen, Cheng Lian, Xiangwen Gao and Chunzhong Li from East China University of Science and Technology (Shanghai, China) and Oxford University (UK) have now begun to overcome this challenge. Their inspiration comes from small lizards. Anolis lizards live on land but can also breathe underwater while swimming in search of food. To do this, they generate air that is applied to the scales on their heads.
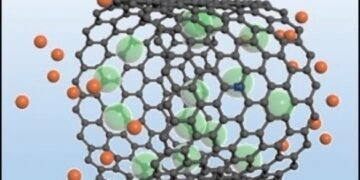
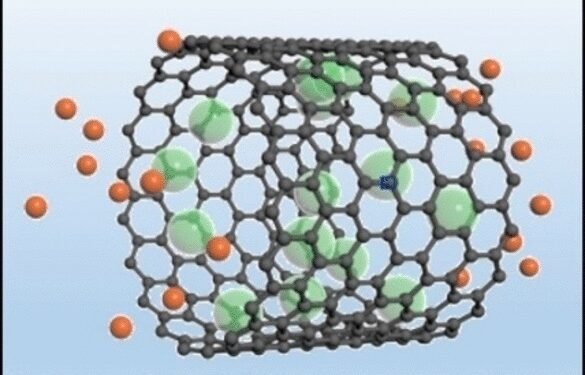
Under water, they breathe bubbles repeatedly. Newly developed electrodes made of porous carbon materials (preferably carbon nanotubes with hollow walls of about 3 nm in diameter) can also maintain a gas layer when placed in a table salt solution as an electrolyte. The gas used is not air, but chlorine.
During charging and recharging, this electrode undergoes a redox reaction in addition to the usual charge separation for hard electrodes. When charging, the electrode transfers electrons to chlorine gas, reducing chlorine to chloride ions, which enter the solution – the electrode “exhales”. When it is released, the chloride ion is oxidized to chlorine, which returns the gas to the pores of the electrode – the electrode “inhales”.
Using different methods of analysis, the group showed that no chlorine gas was coming out of the electrode. Fast reduction/oxidation and fast transfer in the thin gas layer dramatically increases the energy density of the supercapacitor while maintaining a very high energy density. The capacity remains at the same high level even after thousands of cycles.