A clean system that separates impurities from air and water is necessary to support life on Earth. To achieve this, carbonaceous materials have long been used to deodorize, isolate and remove harmful anionic impurities from the media. Until now, the exact mechanism of carbon that makes water clean is still a mystery. Also, it is not known whether the water solution in which the carbonaceous is added is acidic, alkaline or neutral. To fill these gaps, the researchers of Dr. Led by Takahiro Ohkubo, Associate Professor in the Department of Chemistry, Faculty of Science and Technology, Okayama University, Japan, the basic structure of carbon nanopores (SWCNTs to replace anionic impurities) using anions was investigated.
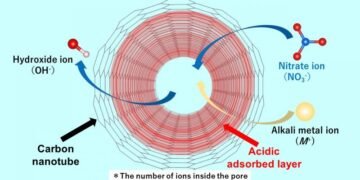
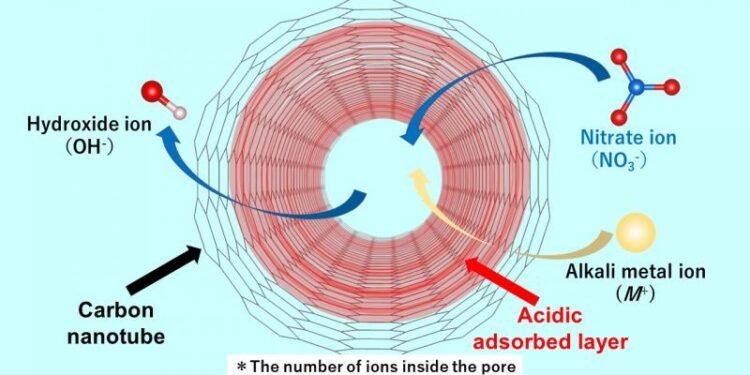
In a recent paper uploaded September 16, 2022 and published in Volume 629 Part B of the journal Colloid and Interface Science, researchers report using Raman spectroscopic equipment to investigate the absorption of nitrate ions through a cylindrical pore of single-walled carbon nanotubes (SWCNTs). Dr. Ohkubo and his colleagues succeeded in discovering the mechanism by which an acid layer is formed along the pore wall. It turns out that when an aqueous solution containing ions enters the carbonaceous material, even if the aqueous solution is neutral, an acidic layer containing protons is created that stabilizes the situation. Speaking about the innovation and the main nature of their work, Dr. Ohkubo says: “Until now, there are no reports that there are acid-producing compounds in nanotubes of carbonaceous materials.
The research team, which includes Dr. Nobuyuki Takeyasu, an assistant professor in the same department at Okayama University, found that the acid layer makes the diffusion of the negative nitrate anion negative, while the number of nitrate ions is greater than that. of cations or positively charged groups. Also, hydroxide ions are produced as counter ions. Hydroxide ions in SWCNTs (SWCNTs to replace anionic impurities) are used to replace the anions in the bulk solution, making the aqueous solution alkaline. The team investigated anion adsorption using several alkali metal nitrates, including solutions of lithium nitrate, sodium nitrate, rubidium nitrate and cesium nitrate. They found that more nitrate ions diffuse than iron ions. The proton diffusion rate is almost the same regardless of the type of alkali ion used. Dr. Says Ohkubo: “The acid layer in the pore can activate the nitrate anion species because of both the strong binding by the pore and the strong interaction between the layer and the anion.”
The results are indeed an important step towards the design and development of carbon nanotubes suitable for ion adsorption in water and clean air. The purification process presented in this research is a new model that explains the alkalinity of aqueous solutions, which has been a mystery until now. The researchers found that the results of their study emphasize that it is necessary to remove water before use when the non-ionized materials are held by the carbonaceous materials. Another significant aspect of this study is the demonstration that the interface of nanomaterials is a new area of chemical reactions, which can lead to further experiments. Taken together, this work brings our understanding of the mechanism of anion diffusion through carbon to another level, paving the way for new carbon nanotubes as efficient purifiers.