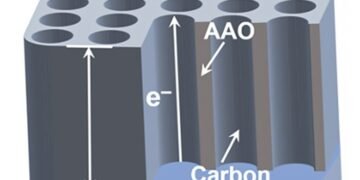
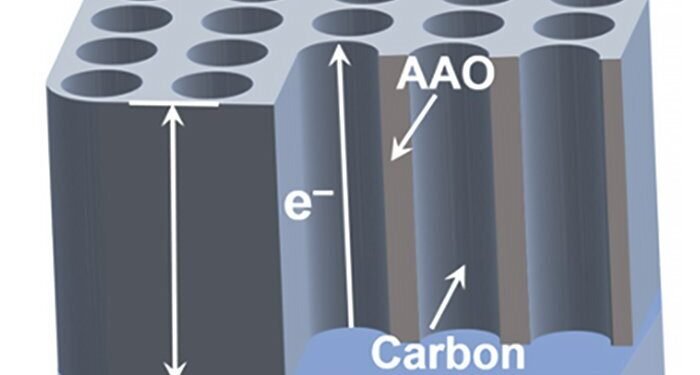
Researchers from Tohoku University and Tsinghua University have developed a breakthrough membrane electrode design that promises to revolutionize basic electrochemical research. This new electrode, produced by a well-designed process, features many large carbon nanotubes (gCNTs) ordered in a nanoporous membrane, opening new opportunities for energy storage and electrochemical studies.
The main breakthrough is in the construction of this new electrode. The researchers developed a process for yellow carbon coating and anodic aluminum oxide (AAO) formed on the aluminum substrate, removing the barrier. The resulting coated carbon layer features vertically aligned gCNTs with nanopores ranging from 10 to 200 nm in diameter and 2 μm to 90 μm in length, encapsulating small electrolyte molecules and large related bio-factors such as enzymes and exosomes. Unlike traditional composite electrodes, this independent model removes the contact between the parts, making the contact resistance small – which is important for defining the corresponding electrochemical characteristics.
“The power of this model electrode is very impressive,” Dr. Zheng-Ze Pan, one of the study’s corresponding authors said. “Using an electrode membrane model with its limited nanopore size, we can gain insight into the complex electrochemical processes that occur in porous carbon electrodes, and their relationship to nanopore size.”
In addition, gCNTs contain sheets of graphene with low crystallinity, offering unprecedented opportunities for electrical reconstruction with low carbon walls. Through experimental experiments and the use of a desorption system with a controlled internal temperature, the researchers built a rectifier of the same size as the thin carbon crystal walls, allowing detailed simulations to be performed.
Dr. Alex Aziz, who led the simulation part of the research, pointed out: “Our high resolution provides a unique space for measuring electron transitions in amorphous carbon, shedding light on the complex processes that govern behavior their electricity.”
Source: Tohoku University