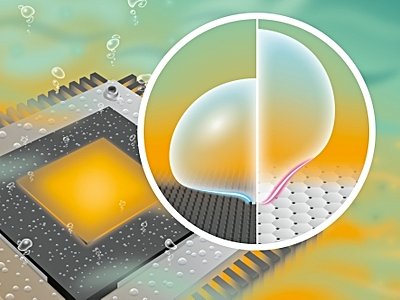
When the liquid evaporates from the container, small bubbles of vapor form at the bottom and rise, transferring heat to the system. How these tiny bubbles grow and burst was previously unknown. A group of German-Chinese researchers under the leadership of the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) have now succeeded in extending this understanding (A nanoscale view of bubble formation) in a fundamental way. Thanks to computer simulations, experts have succeeded in modeling the behavior of particles at the liquid-gas interface at the nanometer scale, allowing them to describe the boiling process with precision. This discovery can be applied to microprocessor cooling systems in the future, or for the production of carbon-neutral hydrogen, known as green hydrogen, as the group reported in the “Journal of Colloid and Interface Science (DOI : 10.1016/j.jcis.2022.10).062).
The amount of water droplets or bubbles falling on the surface depends on the shape and size of the surface. For example, spherical drops are formed on hydrophobic material, with little surface contact with the base. In a hydrophilic material, however, water causes the formation of a flat deposit – the solid interface of the liquid becomes larger. Young-Laplace groups can describe such systems in terms of systems. This equation gives a contact angle that reflects the behavior of water droplets on a surface: a large angle indicates negative wetting, while a small angle indicates good wetting. When steam bubbles form on the walls in boiling water, a thin film of water – which is invisible – remains at the bottom. This movie determines how the bubble grows and how it clears the wall. The contact section also plays an important role in this.
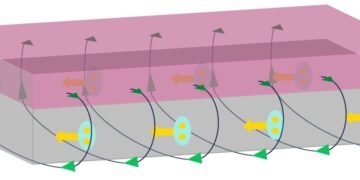
The underlying concept is based on a relatively simple approach. “It takes into account both the pressure of the liquid outside and the pressure of the air inside the bubble,” explained Professor Uwe Hampel, head of hot water exchange at HZDR. “Then there is capillary pressure, which is created by the curvature of the bubble surface.” Recently, however, experimental methods using laser measurements have shown that this established theory fails for small water droplets and bubbles: at the nanoscale, the measured contact angles in some cases deviate significantly from predictions .
Complex interactions of molecules
To solve this problem, a German-Chinese research team arose to revise the concept. To do this, they looked more closely at the processes that occur when water boils. “We looked in detail at the interface behavior of particles,” HZDR researcher Dr. Wei Ding explained. “Then we used computers to simulate the interactions between these molecules.” In doing so, the research group discovered a major difference from previous methods: the forces acting between particles do not just add up in a linear fashion. Instead, the relationship is more complex, resulting in a variety of unintended consequences. These are the exact effects that experts consider in their new expanded view. “Our hypothesis provides a good explanation for the results obtained in recent experiments,” Ding said excitedly. “Now we have a more accurate understanding of the behavior of small vapor droplets and bubbles.”
In addition to completing our understanding of the mechanism of action, the results also hold promise for progress in many technical areas, such as microelectronics. Here, the generators are now so powerful that they generate heat, which must be removed by the cooling process. Uwe Hampel said, “There is an idea for spreading this fire by boiling water.” “With our new approach, we will be able to determine the conditions under which rising vapor bubbles can effectively disperse heat.” Equilibrium can also help make the fuel in nuclear reactors more efficient than in the past.
Hydrogen production is efficient
Another possible application is the electrolysis of water to produce carbon-neutral hydrogen, called green hydrogen. Unexpected gas bubbles form on the surface of the electrolyser during water separation. With this new theory, it seems conceivable that these bubbles can be affected more precisely than before, allowing for more efficient electrolysis in the future. The key to all these applications can be based on the selection and design of the appropriate material. Wei Ding explained, “adding nanogrooves to the surface, for example, can accelerate the release of gas during boiling.” “Thanks to our new ideas, such a structure can be improved significantly – our work is working.”