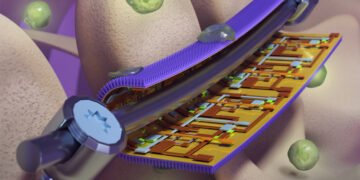
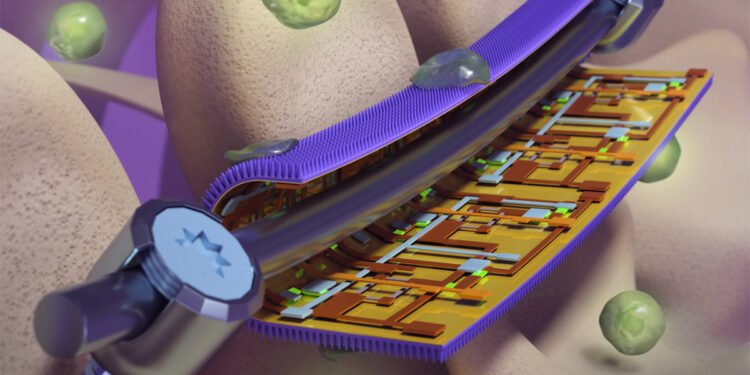
A new ‘smart’ mask developed for surgical orthopedic implants can monitor pressure and device to provide early warning of implant failure while killing infection-causing bacteria, researchers from the University of Illinois at Urbana-Champaign is telling. The masks include flexible sensors with nanostructured antibacterial surfaces derived from the wings of dragonflies and cicadas.
In a new study published in the journal Science Advances, a group of researchers found that the coating prevented the infection of live mice and that was produced in a commercial model in sheep’s bones to warn of various failures of implantation.
“It is a combination of bio-inspired nanomaterial design and flexible electronics to combat this complex and long-term biomedical problem,” said study leader Qing Cao, U. of I. Professor of Materials Science and Engineering.
Infection and device failure are major problems in orthopedic implants, each affecting up to 10% of patients, Cao said. Many methods have been tried to fight this disease, but all of them have limitations, he said: Biofilms can still form on waterproof surfaces, and the coating of antibiotics or drugs broadcast environment. The body is less active against resistant types of bacteria.
Inspired by the naturally antibacterial wings of the criminal and dragonflies, the Illinois team has created a thin sheet modeled with nanometric pillars like those found on the wings of insects. When a bacterial cell tries to bind to the leaf, the pillars puncture the cell wall, killing it.
“Using a chemical method to kill bacteria allows us to avoid many of the problems associated with chemical methods, while it gives us the flexibility to put the coating on the implant,” said the professor of pathobiology. Gee Lau, a research writer.

On the back of the nanostructured sheet, where it contacts the embedded device, the researchers added a highly flexible electronic sensor to monitor the shape. Researchers say it can help doctors monitor the progress of each patient’s treatment, guide their rehabilitation to reduce recovery time and reduce risk, and repair or replace devices before they reach the limit. of failure, researchers said.
A group of engineers teamed up with Professor of Veterinary Clinical Medicine Annette McCoy to test their device. They put his papers into living mice and examined them for any infection, even when the bacteria started. They also applied the mask to a commercially available back implant and checked the stress on the implant in the sheep bone under normal load for the diagnosis of device failure. The cover did both jobs well.
This electronic prototype requires wires, but the researchers plan to create wireless power and wireless data communications for their coating, a crucial step for clinical applications, Cao said. They are also working on improving the mass production of nanopillar-textured bacterial sheets.
“These types of bacterial coatings have many potential applications, and since ours uses a process, it has the potential for chemicals or heavy metal ions – as currently used in coatings antimicrobial coating – will be destroyed,” said Cao.
Source: University of Illinois at Urbana-Champaign.