Despite the wide application of radiation in diagnostics and treatments, there is still no magic material to protect people from radiation exposure. Though Amifostine is developed as a radioprotectant, the FDA approved its application only for the protection of salivary glands. Also it requires repetitive injections to be effective and it can pose severe health threats of systemic toxicity and complications when applied to those under total body irradiation conditions.
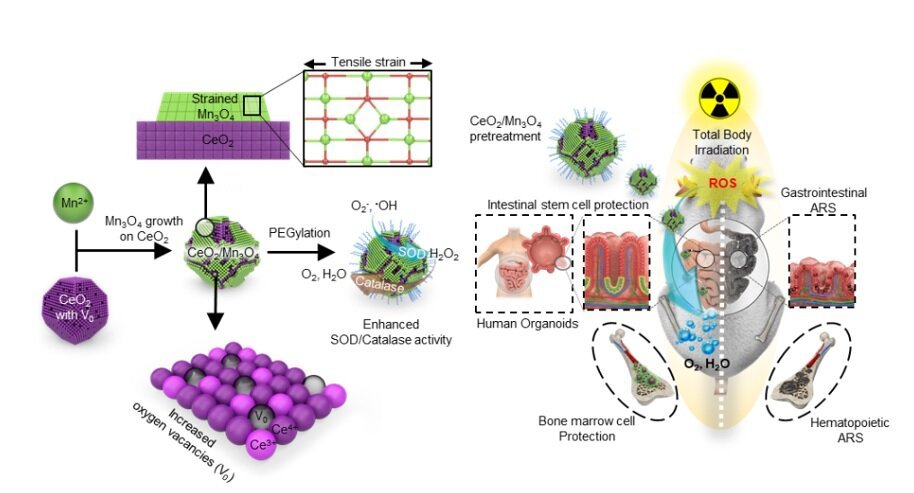
Researchers at the Center for Nanoparticle Research, within the Institute for Basic Science (IBS, South Korea) in collaboration with their colleagues at Seoul National University, School of Dentistry and Dental Research Institute, have reported a highly effective and safe nanocrystal to combat dangerous doses of radiation. By growing manganese oxide (Mn3O4) nanocrystals on top of Cerium oxide (CeO2) nanocrystals, the research team boosted the catalytic activity of the CeO2/Mn3O4 nanocrystals in their ability to stave off side effects of deadly radiation.
“Excessive reactive oxygen species (ROS) are found in a number of major diseases including sepsis, cancer, cardiovascular disease, and Parkinson’s disease, just to name a few,” says Hyeon Taeghwan, director of the IBS Center for Nanoparticle Research (Seoul National University Distinguished Professor). “A powerful antioxidant that can work at low doses only can ensure sustainable applications of radiation in medical, industrial and military settings and more. These new CeO2/Mn3O4 hetero-structured nanocrystals are five times stronger than when CeO2 or Mn3O4 does the job alone,” notes Director Hyeon.

When our body is exposed to high levels of radiation, a massive amount of ROS are generated within milliseconds due to the decomposition of water molecules. These ROS severely damage cells, eventually leading to their death. The research team looked to CeO2 and Mn3O4 nanoparticles for their outstanding ROS scavenging abilities. The challenge was how to apply these antioxidant nanomaterials in a safe and economic way. Though effective, CeO2 and Mn3O4 nanoparticles can remove ROS only in high doses. They are also rare materials and difficult to obtain.
The researchers drew on the approach usually taken in the field of catalysis: stacking nanoparticles with different lattice parameters results in surface strain and increases oxygen vacancies on the surface of the nanocrystal. “The synergistic effect of the strain generated on Mn3O4 and the increased oxygen vacancy on the CeO2 surface improved the surface binding affinity of the ROS, boosting the catalytic activity of the nanocrystals,” Han Sang Ihn, the first author of the study, explains.
“Strain engineering of nanocrystals, mainly studied in the field of catalysts, has now been extended to the medical field to protect cells from ROS-related diseases,” says Cho Min Gee, the co-first author of the study.

The research team checked the safety, as well as effectiveness of these new antioxidant nanocrystals. Molecular dynamics are analyzed using acute radiation model of human intestinal organoids. “Organoids pretreated with the CeO2/Mn3O4 nanocrystals expressed more genes that were related to proliferation and maintenance of intestinal stem cells and fewer cell-death genes, compared with the no-pretreatment group,” explains Sang-woo Lee, the first co-author of the study. In a mouse study, the CeO2/Mn3O4 nanocrystals significantly increased the survival rate of the animals to 67% with only a very small dose (1/360 of Amifostine injection dose) and decreased the oxidative stress to internal organs, circulation, and bone marrow cells, without any significant signs of toxicity
“To ensure a safe and wide application of a radioprotectant in the clinic, the key is to maintain high catalytic efficacy in low doses. These CeO2/Mn3O4 nanocrystals prove their powerful antioxidant effects to protect our whole body effectively just in small doses,” says Park Kyungpyo, professor at Department of Dentistry, Seoul National University.


































