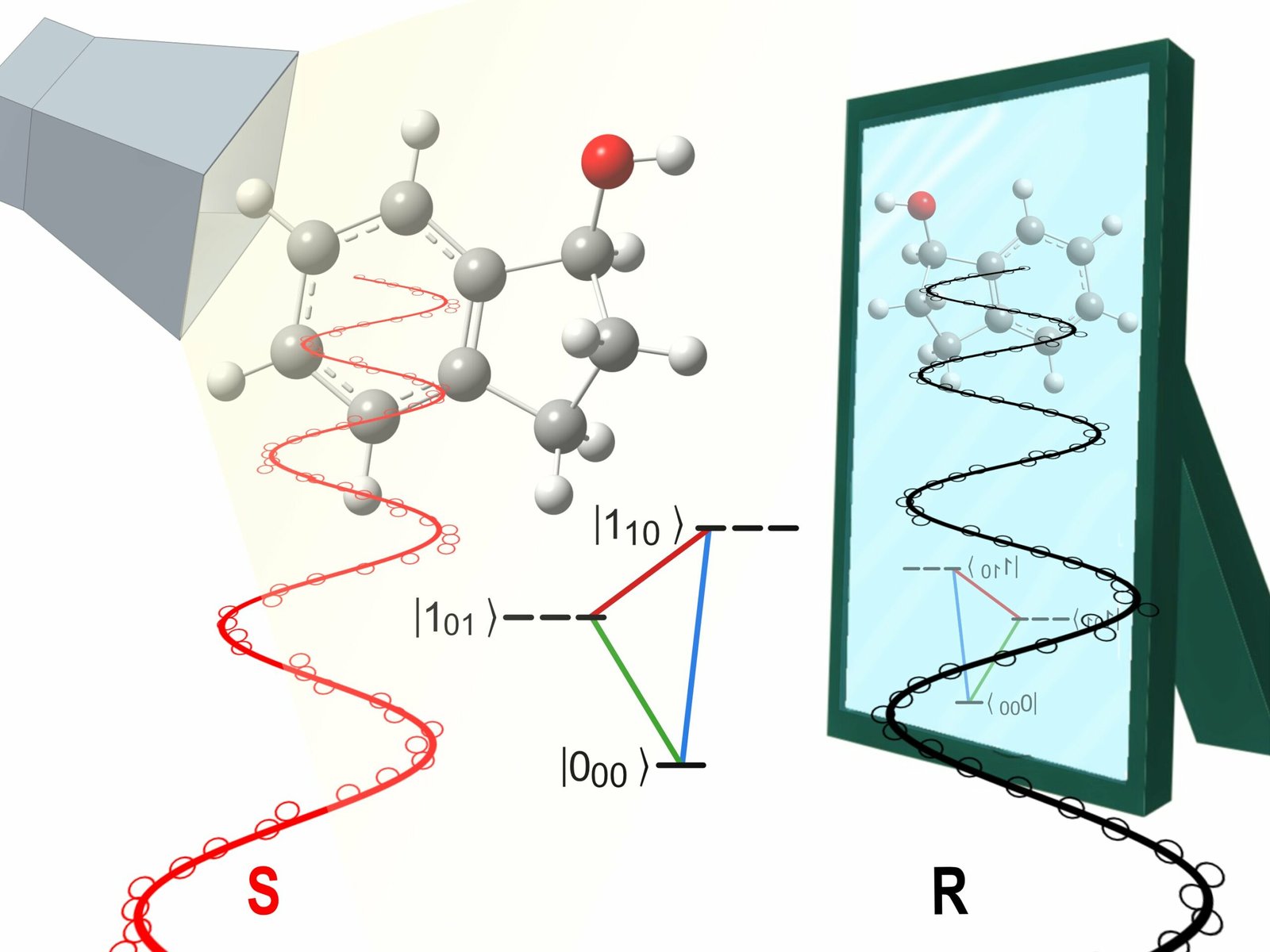
However, chirality, although not unique in the world of molecules, is a special feature. If the molecule is chiral (from the Greek word chiros = hand), then it is in two mirror versions (Check pictures of chemical glass), which are very similar but not identical with both hands, which can be folded, but at the same time not put on top of each other.
That is why we are talking about the molecule of the right and left hand, or an enantiomer, which in Greek means “opposite form”. An international group of scientists from the Fritz Haber Institute of the Max Planck Society and Prokhorov General Institute of Physics of the Russian Academy of Sciences has found a way to dissolve these molecules separately(Check pictures of chemical glass).
Given that chiral molecules are very similar to each other, this is a real challenge. “The trick is to expose them to electromagnetic radiation in such a way that only one ‘hand’, the enantiomer, can react. This allows us to specifically control right-handed or left-handed molecules and learn more about them,” he said. Dr. Sandra Eibenberger-Arias, Head of the Controlled Molecules Group at the Fritz-Haber Institute.
This learning is important because enantiomers sometimes have very different biological and chemical properties, for which explanations are sought. Think, for example, of the chiral molecule carvone: one “hand” smells like mint, the other corn. Or the famous sedative thalidomide, named after its active ingredient, is a chiral molecule: while one form has the desired sedative effect, the other causes birth defects. The Eibenberger-Arias group studied the physical properties of chiral molecules. “The theory predicts a small difference in energy between the two enantiomers due to the so-called parity violation. However, this has not yet been proven in the experiment,” explained JuHyeon Lee of the Fritz-Haber Institute, the first author of the book. published results that appeared in the journal Physical Review Letters. However, an intelligent combination of different methods brings the team of scientists closer to achieving this goal. They irradiate chiral molecules in the gas phase with UV radiation and microwaves. As a result, the clockwise and left-handed molecules are placed in different rotational states as the microwave radiation changes. Researchers thus have more control than ever over which “hand” is in a rotating state. For the first time, they also compared experimental results with accurate predictions from theory, leading to a better understanding of the underlying physical effects. Although complete separation of the enantiomers in this way has not yet been achieved, it is noteworthy that they can be successfully controlled in the first place. This is in contrast to the commonly used simplified description that they have the same physical properties. “If that’s the case, we can’t control enantiomers by physical methods,” says Sandra Eibenberger-Arias. An international team of three female and three male scientists has laid a good foundation for subsequent experiments and possibly experimental evidence of parity violations. This is a milestone for basic research – and for all future applications.