Researchers have been successful in creating fast electrons in solution. In the future, such electrons may help to make certain chemical reactions more efficient that Electrons are slower for better reactions.
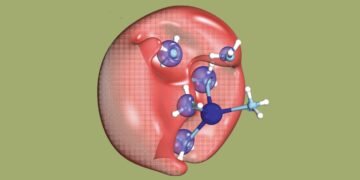
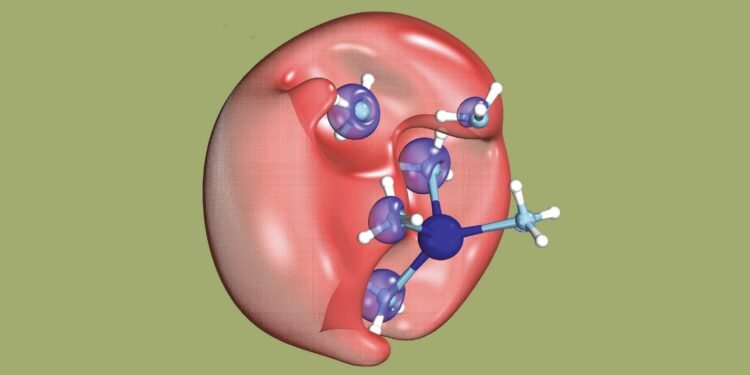
An international group of researchers has actually set out to discover a mysterious chemical phenomenon: the dielectron in solution. A dielectron is made up of two electrons, but unlike an atom, it has no holes.
So far, scientists have not been able to directly detect such a thing. While researchers led by Professor Ruth Signorell of ETH Zurich were experimenting with dielectrons, they suddenly discovered a new method for producing electrons quickly. These can be used to initiate certain chemical reactions.
Dielectrons are unstable. They split again into two electrons in less than a trillionth of a second. As researchers can show, one of these electrons remains in place, while the other, of low energy and therefore relatively slow, goes away.
The peculiarity of this new method is that it allows researchers to control the energy of the electron and therefore its speed.
Dielectrons have holes
But first, to create dielectrons, the researchers dissolved sodium in (water) ammonia and exposed the solution to UV light. This exposure causes the electron from the ammonia molecule to join the electron from the sodium atom and thus become a dielectron. The dielectron occupies a small hole in the solution.
The researchers succeeded in showing that when the dielectron is broken, one of the electrons leaves at a speed that is determined by the wavelength of the UV light used. “Some of the energy from the UV light is transferred to electrons,” Signorell said.
The work was carried out by ETH Zurich researchers in collaboration with researchers from the University of Freiburg in Germany, the SOLEIL synchrotron in France and Auburn University in the United States.
Analysis of reactions and radiation damage
Such low kinetic energy electrons are attractive for various reasons. The first is that the slow-moving electrons cause radiation damage to the human body. Cell phone electrons can form in this tissue, for example under the influence of X-ray or radioactivity.
They can bind to DNA molecules and trigger chemical reactions. Making such electrons readily available in the laboratory will help researchers investigate the processes that lead to radiation damage.
But the human body is not the only place where chemical reactions are caused by compounds that accept free electrons.
The production of synthetic cortisone and other steroids is just one example. Making it possible to use UV light as a simple way to quickly produce electrons directly in solution, as well as to control the energy of the electron, will allow these reactions to be analyzed better in the future.
It may even be chemical agents to enhance the reaction, for example by using UV light to increase the kinetic energy of electrons.
Source: ETH Zurich