In the world’s most powerful X-ray facility, scientists can analyze samples as small as 10,000 atoms. Smaller sizes are very difficult to achieve, but one company with many companies failed at one atom and now for the First Time Atoms with X-rays examined.
Saw Wai Hla, a scientist at the US Department of Energy’s (DOE) Argonne National Laboratory and a professor at Ohio University, said, “X-rays are used everywhere, including security research, medical imaging and diagnostics is the point.” “But since the discovery of X-rays in 1895, scientists have not been able to detect and analyze a single atom. Scientists have thought they would be able to do this for decades. Now we can.”
As reported in Nature, scientists from Argonne and various universities report that they can define the elemental nature and chemical properties of an atom using X-ray beams. This new power will have an impact on basic research in many scientific processes and the development of new technologies.
The results of the X-rays provide a kind of fingerprint for the type of material in the material. For example, NASA’s Curiosity rover collected small pieces of sand from the Martí planets and determined through X-ray analysis that their contents resemble volcanic soil in Hawaii.
Using powerful X-ray machines called synchrotron light sources, scientists can analyze samples as small as a billionth of a gram. These samples contain about 10,000 atoms. The small size proved to be very difficult to achieve, but in a dramatic leap, the team managed to reduce their findings to a single atom.
“The term is being interpreted interchangeably, but I think this discovery is really a breakthrough,” Hla said. “I am happy that I can sleep as I think that batteries can be used in the development of microelectronic devices and even in environmental research and health.”
To define an atom as having X-rays, it must be in the same type of atom. To do this, the team first wrapped an atom of iron in a nanometer-sized object that has different components.
They then took the sample with powerful X-ray light from Argonne’s Advanced Photon Source (APS). The team detected single atoms in a sample and beamline (XTIP) shared by APS and the Center for Nanoscale Materials (CNM). Both are owned by the DOE Office of Science and Argonne. The light includes scanning tunneling microscopy (STM).
Volker Rose, a physicist at APS and CNM said, “The DOE Career Research Program Award that I received in 2012 allowed me to create a group of enthusiastic scientists and engineers. “Together, we developed and built this microscope at the XTIP beamline with additional funding from the DOE.”
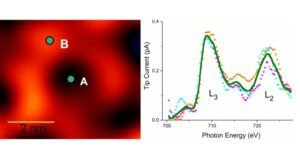
A beam of photons from the X-ray beam strikes the sample, causing it to release electrons. Less than one nanometer above the surface of the sample, the STM probe collects electrical signals due to the emitted electrons. Specimens (plots of current versus photon energy) are “fingerprints” for elements in the periodic table. Everything has a unique fingerprint. By examining the surface of the sample, scientists can thus determine which part of an atom is exactly where it is.
There is something else. They can also determine the chemical state of atoms from the same spectrum. Chemical conditions are related to the fact that atoms can lose a certain number of electrons; for example, iron can lose two, three or four electrons. The chemical state reflects the number of missing electrons and it is important for scientists to know this because it affects the physical, chemical and electronic properties of atoms.
To demonstrate the new ability’s wide range, the team successfully replicated the same X-ray probe with terbium. Rare earths are important for microelectronics, batteries, aerospace engineering and more. This process also applies to other things than metals. By knowing the properties of individual atoms, scientists can use them in new ways.
“Being able to study one atom at a time will revolutionize X-ray technology at an unprecedented level, from quantum information technology to environmental and medical research,” Hla said.
Source: Argonne National Laboratory




































