Quantum dots are nanoscale crystals that can emit light of different colors. Display devices based on quantum dots promise greater energy efficiency, brightness and color purity than previous display generations. Of the three colors commonly used to display color images – red, green and blue – the latter has proven to be difficult to produce. A new technique (From nowhere) based on self-organizing chemical structures provides a solution, as well as an optical imaging technique to visualize blue quantum dots has proven useful for their production and analysis. Look closely at your device’s screen, and you should be able to see the individual images, the pixels, that make up the image. Pixels can appear in almost any color, but they’re not necessarily the smallest thing on your screen since they’re usually made up of tiny red, green, and blue pixels. The different energies of these tiny pixels give each pixel the appearance of a single color from a palette of billions. Sub-pixel technology has evolved since the days of the first color TV, and there are many options available now. But the next big improvement will be so-called quantum dot light-emitting diodes, or QD-LEDs.
Displays based on QD-LEDs already exist, but the technology is still developing, and the current options have some drawbacks, especially regarding the blue sub-pixels they contain. Of the three primary colors, the blue sub-pixels are the most important. Through a process called down-conversion, blue light is used to produce green and red light. For this reason, blue quantum dots require more controlled physical parameters. This often means that blue quantum dots are complex and expensive to produce, and their quality is critical to any display. And now, a team of researchers led by Professor Eiichi Nakamura from the Department of Chemistry of the University of Tokyo has a solution. “Previous design strategies for blue quantum dots were advanced, taking large quantities of chemicals and putting them through a process to refine them into active materials,” Nakamura said. “Our plan is bottom-up. We rely on our team’s knowledge of self-organizing chemistry to precisely manipulate molecules until they form the desired properties. Think of it like building a house with bricks instead of stone. It’s easy to say well, design the way you want, and it’s very efficient and cost-effective.

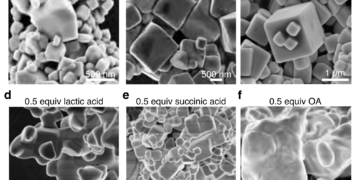
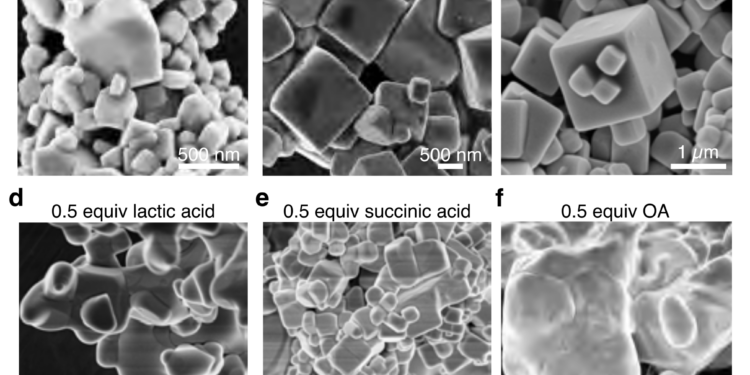
But it’s not just how Nakamura’s team created their blue quantum dots that is unique; when exposed to ultraviolet light, it produces almost perfect blue light, according to the international standard for measuring color accuracy, known as BT.2020. This is due to the unique chemical composition of their stains, a hybrid mixture of organic and inorganic compounds including lead perovskite, malic acid and oleylamine. But only by personal arrangement can these be put into the desired form, which is a cube of 64 lead atoms, four on each side.
“Surprisingly, one of our biggest problems was finding out that malic acid is a key part of our chemical complex. It took more than a year to use different methods to find it, “Nakamura said. “Perhaps, it is not surprising that our next major challenge is to determine the structure of the blue quantum dot. At 2.4 nanometers, 190 times smaller than the wavelength of the blue light we want to produce, the structure of quantum dots cannot be produced by ordinary means. So we turned to a visual tool that some of our team members know as SMART-EM, or “cinematic chemistry” as we like to call it. »
Cinematic Chemistry is the introduction of electron microscope imaging that is more suited to videography than photography. To capture the structural details of the blue quantum dot, this is important, because the nanocrystal is really a solid thing, so each image of it will be only a small part of its story. Unfortunately, the blue quantum dot also has a short lifespan, although this is expected, and the team is looking to improve its stability using industrial collaboration.