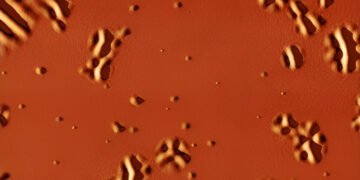
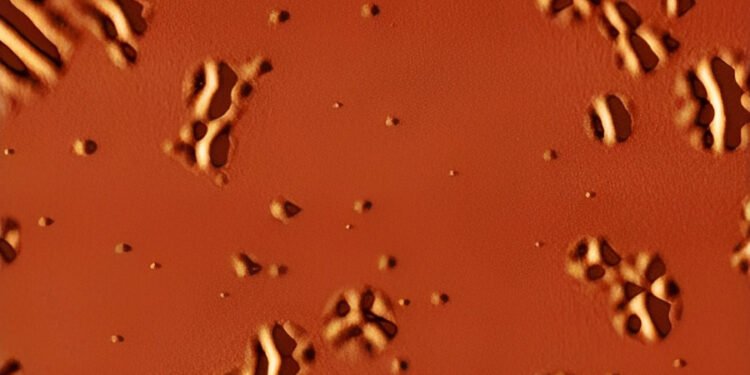
First observation on the nanometric scale of the transition of glass in liquid with increasing temperature. Glass is a solid material with such a poor structure that it can be considered a liquid of exceptionally high viscosity. It is found in transparent windows and stained glass, in television screens and mobile devices, in optical fibers, in industrial plastics, but also in the state of proteins, cellular parts and living tissues when frozen for preservation.
Although it is common, it is difficult to create concepts and models that can explain their behavior in detail. The process by which liquid water cools and turns into glass, and conversely, how glass turns into liquid when heated, known as the “glass transition“, is not fully understood.
Scientists still do not know if it is a change of time and the glass can be considered as a thermodynamic situation different from liquid to solid state; or if the glass is just a supercooled liquid – cooled below temperature but still retaining the properties of water – whose atoms or molecules have less mobility.
One of the difficulties in understanding this process is the challenge of visualizing it under the microscope with full resolution, since the structure of the cold water in the glass is not detectable.
A group of researchers from the Department of Physics of the Universitat Autònoma de Barcelona (UAB) and the Catalan Institute of Nanosciences and Nanotechnologies (ICN2), with the participation of UPC and IMB-CNM-CSIC, presented a new method that allows. look directly under the microscope what happens to the glass when it heats up the glass changes temperature, which is called the process of “relaxation” that makes it liquid. Researchers are working on ultra-stable organic glass, which is prepared by heating.
They are more expensive and exhibit more kinetic and thermodynamic stability than glass obtained directly from liquid. Unlike the common glass which, as it is seen now, turns all over the world in a liquid state, without a clear distinction between the different areas of the material, This sparkling glass turns to a cool liquid state in the same way as a solid crystal when they enter it. water conditions, and the zoning of water areas is increasing.
This is a process that has been vaguely described by nanocalorimetric measurements and has only been observed in computer models. Cristian Rodriguez Tinoco, a researcher at UAB says, “In the past, it has been found that these species in the area of water production show a special separation between them when they interact with Glass is strong, but this is not immediately obvious.” ICN2.
A new technique developed to achieve this transition is sandwiching a stronger glass between two glasses with a higher transition temperature. When this rare glass layer is heated above its transition temperature, the stability that occurs on it is transferred to the upper surface of the sandwich and can be seen directly with an atomic microscope.
“These are small movements and compressions, of the order of a few nanometers at the beginning of the change, but large enough to be measured directly with a microscope of this type, which examines the condition of the surface deformation that – come out on top. a revolutionary situation,” explains PhD student Marta Ruiz Ruiz.
The structure makes it possible to follow the rotation of the glass immediately. It makes it possible to define the strength of the relaxation process in ultra-stable crystals towards liquid cooling by measuring the distance between the emerging liquid sections, where you see the surface deformation and its change over time and – go.
In this way, it will be possible to show how large these distances are between the liquid zones in this type of glass, and the correlation of these distances with the period of time of the object, as predicted by computer model. “The microscopic details we obtained allowed for the first comparison between computer models and physical reality.
We believe that this method will also be very useful for exploring the transition of glass in a short period of time in space, which will lead to a better understanding of the changes in the hard glass produced by liquid cooling”, Javier Rodríguez Viejo, researcher at UAB concludes. ICN2.
Source: Universitat Autònoma de Barcelona