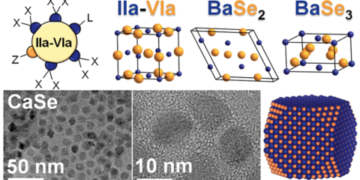
Research and synthesis of new materials can lead to environmentally sustainable products such as solar panels and light emitting diodes (LED). Scientists from Ames National Laboratory and Iowa State University have developed (Important research leads to the understanding of new optics) a colloidal synthesis method for alkaline earth chalcogenides. This method allows them to control the size of the nanocrystals and the material. They can also study the surface chemistry of the nanocrystals and determine the purity and optical properties of the compounds involved.
Alkaline-earth chalcogenides are a type of semiconductor of growing interest in scientists. They have various applications such as bioimaging, LEDs, and thermal sensors. These compounds can also be used as optical materials such as perovskites, which convert light into energy.
According to Javier Vela, Ames Laboratory Scientist and John D. Corbett Professor of Chemistry at Iowa State University, one of the reasons (Important research leads to the understanding of new optics) these new materials are so exciting is that they “are made up of materials that are abundant in terrestrial species, making them a good alternative compared to conventionally used toxic or expensive semiconductors.
Vela explained that the most commonly used semiconductors contain lead or cadmium, two substances that are harmful to human health and the environment. In addition, the most popular method used by scientists to produce these materials involves solid state reactions. “These reactions usually occur at high temperatures (over 900°C or 1652°F) and require reaction times that can last anywhere from days to weeks,” he said.
On the other hand, Vela describes the “chemical solution (Colloidal) can pick up temperature (less than 300 ° C or 572 ° F) and a small reaction is short.” Therefore, Colloidal methods used from Vala membership requires that time to pack.
The Vela team found that colloidal procedures have used them to work with nanocrytry. The size of the nanocrystals is important because it determines the optical properties of certain materials. Vela explained that by changing the size of the particles, scientists can affect the ability of the material to receive light. “This means we can create materials that are suitable for applications just by changing the size of the nanocrystals,” he said.
According to Vela, the group’s first goal is to create semiconducting alkaline-earth chalcogenide perovskites, because they can be used in solar devices. However, to achieve this objective, they needed a deeper understanding of the fundamental chemistry of the Chalcogends of the alkaline earth. They therefore chose to focus on these binary materials instead.
Vela said that their research fills the need to improve the understanding of scientists about photovoltaic, luminescent and thermoelectric materials that are produced in the earth, which are non-toxic. He said: “We hope that our development and this work will eventually contribute to the synthesis of complex nanomaterials, such as alkaline earth chalcogenide perovskites.”