Medical microrobots can help doctors better treat and prevent disease. However, most of these devices are made of synthetic materials that trigger immune responses in vivo. Now, for the first time, researchers reporting to ACS Central Science are using lasers to control neutrophils — a type of white blood cell — as well as a natural, biocompatible microrobot in live fish. These “netobots” perform many tasks, suggesting that in the future they will be able to deliver drugs to precise locations in the body.
The microrobots now being developed for medical use require injections as well as ingestion of capsules to enter an animal or human. However, scientists have found that these microscopic substances often trigger immune responses in small animals, leading to the removal of the microrobots from the body before they can do their job. Using cells already in the body, such as neutrophils, may be a less invasive alternative to administering drugs that do not suppress the immune system.
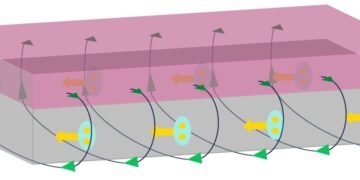
These white blood cells naturally capture nanoparticles and dead red blood cells and can migrate through blood vessels to surrounding tissues, making them good candidates to become microrobots. In the past, researchers used lasers to guide neutrophils into laboratory dishes and activate them as “neutrophils.” However, information on whether this method works in live animals is lacking. Xianchuang Zheng, Baojun Li and colleagues wanted to demonstrate the possibility of light-controlled neutrobots in animals with live zebrafish.
The researchers manipulated and edited neutrophils in zebrafish tails using focusing laser beams as remote optical tweezers. The light-powered microrobot can move at a speed of 1.3 µm/s, which is three times faster than the natural movement of neutrophils. In their experiments, the researchers used optical tweezers to precisely and actively monitor the functions that neutrophils perform as part of the immune system.
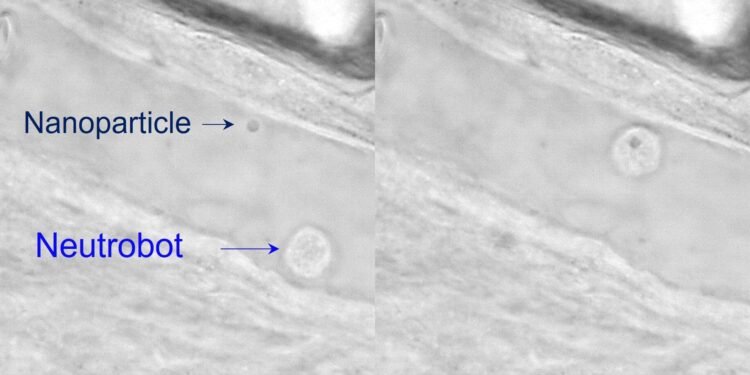
For example, a neutrobot is transferred through the vessel wall to the surrounding tissue. Another grabbed and carried a plastic nanoparticle, demonstrating its drug-carrying potential. And when the neutrobot is pressed into the red blood cells, it swallows the pieces. Surprisingly, at the same time, another neutrophil, uncontrolled by the laser, attempted to naturally remove the cellular debris. Because they successfully monitored the neutrobots in vivo, the researchers say this study improves the potential for targeted drug delivery and appropriate disease treatment.