To develop cancer drugs, researchers can be able to break hard. Many powerful anti-tumor drugs also cause side effects and may end up doing more harm than good. New Nanotech drug delivery system for Cancer Treatments, Scientists have developed a drug delivery system that targets tumors with remarkable precision.
Now, researchers and doctors from the Stevens Institute of Technology and Hackensack Meridian Health have developed a new drug delivery system that uses gold nanoparticles to deliver drugs to tumors with remarkable precision, while reducing power of influence system.
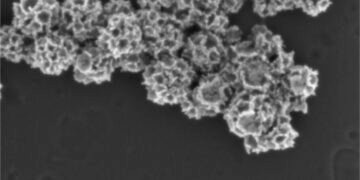
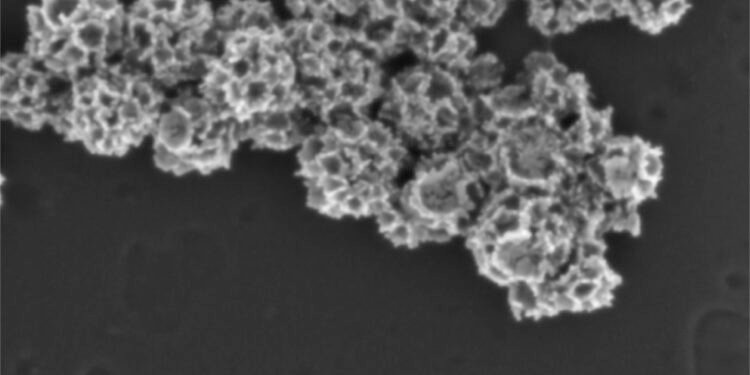
In a paper published in ACS Applied Materials & Interfaces, the team describes the ability to package and seal a drug inside a hollow gold nanoframe, then apply a coating of hyaluronic acid to the surface of the nanoparticle to seal the drug. inside. The result: a stable particle that only releases its payload when it binds to hyaluronic acid receptors on the surface of lymphoma tumors.
“Gold is stable, so it’s the best material for drug delivery,” said Hongjun Wang, professor of biomedical engineering and director of the Semcer Center for Healthcare Innovation at Stevens, who led the research with Johannes Zarkzweski, a doctor at Hackensack. . “Using this system, we are able to deliver drugs more precisely and achieve better clinical outcomes.”
Because the drugs are delivered selectively, with a small drop in the blood, a small amount of drugs can be used to treat the tumor, reducing the risk of toxic effects. In animal studies, the team found no signs of toxicity and no detectable levels of the drug in the blood, despite the fact that the chemicals they provide often cause poisoning in 50% of subjects.
The gold compound continues to circulate in the blood for longer than the floating drug particles, meaning that patients receiving daily treatment can switch to a weekly regimen and it’s two weeks. In animal tests, lymphoma tumors are also more responsive to targeted drug delivery than non-targeted therapies, suggesting that new therapies can be developed to improve outcomes in cancer patients.
The use of gold as a delivery system opens the way for other clinical applications, including the use of gold nanoparticles in tumor research as a contrast agent to make imaging more accurate. They can also be heated by releasing harmless heat energy into the patient’s body, effectively “cooking” it. They can be programmed to deliver their drug load only when triggered by incoming light, providing more control over how and when drugs are delivered.
Nanoparticles can be used to deliver drugs quickly and efficiently, using cheap and scalable chemical processes, which should help reduce the cost of using gold to deliver drugs. “Price is always a concern,” Wang said. “It’s a simple process, and we think the benefits speak for themselves.”
While current studies are focused on validating the use of gold nanoparticle-based drug delivery systems in animals, Wang hopes to see the method developed for testing human cancer patients. “We have shown that it works in animals – now we need to show that it is not safe in humans,” he explained. The pharmaceutical industry has already shown interest in the process, and the FDA has already approved the use of gold in other clinical applications, he said.
Meanwhile, Wang added, the main goal of the research is to develop advanced pore-blocking agents that rely on antibodies to target more difficult-to-treat tumors with high margins. “Our ultimate goal is to use this approach to target multiple myeloma and other cancers that are currently incurable,” Wang said. “Hopefully we can complete the basic research and put this into clinical trials soon.”
Source: Stevens Institute of Technology