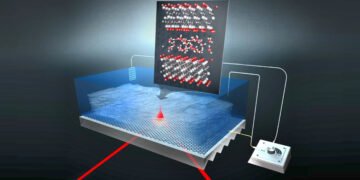
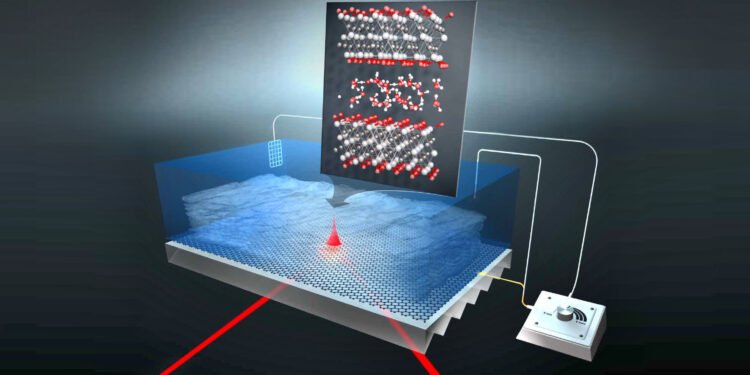
MXenes are able to store large amounts of electrical energy like batteries and chargers and discharge quickly like supercapacitors. They combine both talents and are thus a very interesting class for defense of power. The material is organized as a type of puff pastry, with an MXene film separated by a thin water film. The team from HZB has now studied how protons move in water films (New proton hydration strategy determined) that are sandwiched between materials and allow charge transfer.
Their results have been published in the prestigious journal Nature Communications and may accelerate the optimization of these types of energy storage devices.
One of the biggest challenges for providing climate neutral electricity is electricity conservation. Conventional batteries can hold a lot of energy, but the process of charging and recharging takes time. Supercapacitors, on the other hand, charge very quickly but have limited and limited energy storage. It is only in recent years that a new class of materials combining the advantages of batteries and supercapacitors, called pseudocapacitors, has been considered.
Promising materials: Pseudocapacitors
Among pseudocapacitive materials, the so-called MXenes consist of a large family of carbides and nitrides that appear as promising 2D transition metals. Their shape is like a puff pastry, each layer is separated by a small water film that allows the transfer of charge. Titanium carbide MXenes in particular are conductive and their structure combined with a negative hydrophilic coating provides a unique property of well-charged ions such as protons that can diffuse well. The MXenes used in this study were collected in the group of Professor Yury Gogotsi at Drexel University, USA.
Load transport examined
In recent years, this property has been used to store and release proton energy at unprecedented rates in an acidic environment. However, it remains unclear whether the charges are mainly stored based on the adsorption of protons on the surface of MXene or by desolvation of the proton in the intermediate layer of MXene.
Anticipated retention effects
Due to its two-dimensional geometry, the 2-3 layer film that fits between the MXene layers is expected to handle different protons than the bulk water we get in the past. Although it is assumed that this binding effect plays a role in the rapid diffusion of protons in the MXene material, it is impossible to identify the protons in the MXene electrode during charging and discharging.
Don’t consider vibration patterns
The members of Dr. Tristan Petit led at HZB has succeeded in doing this (New proton hydration strategy determined) for the first time by measuring the vibrational modes of protons excited by infrared light. Postdoctoral researcher Dr Mailis Lounasvuori has developed an electrochemical operando cell that he uses to analyze protons and water in titanium carbide MXenes at BESSY II during the charging and discharging process. In the process, he managed to destroy the unique signature of protons in the water between the MXene layers.
“These vibration patterns are very different from what we would see for protons in a three-dimensional liquid environment,” says Mailis Lounasvuori.
“The fact that water molecules absorb infrared light so much while MXene emits so little light at this high energy makes IR spectroscopy well suited to our question,” said Petit.
A rapid spread is described
This unusual hydration structure, showing that protons are handled by small water molecules under more restriction than in bulk water, suggests that the proton collapse during the interaction between MXene layers can contribute to the storage of pseudo-capacitive energy in an acidic environment.
This can also explain why protons diffuse so quickly in MXene materials, which is related to their discharge/charge time. Besides energy conservation applications, this work shows that MXenes is a good platform to study the basic properties of this restricted chemical species, with other new chemical compounds being discovered.
This method will be applied to other types of cations beyond protons (such as Li + ions) dispersed in the MXene material to reveal a new pseudocapacitive energy storage system within the framework of the ERC start-up grant awarded in 2020.