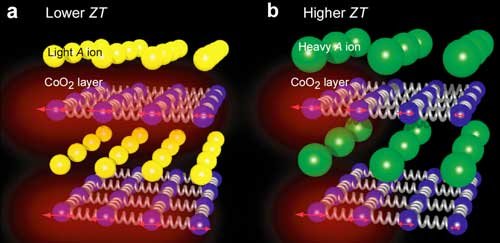
Hypothesis for the ZT improvement of layered cobalt oxide. Ions with greater atomic mass (right) would increase ZT as they suppress thermal conductivity in the cobalt oxide layers. (Image: Yugo Takashima et al, Journal of Materials Chemistry A, October 13, 2020).
Waste heat is a highly promising source of renewable energy; however, the efficiency of using heat to generate energy has historically been much lower than hydroelectric, wind or solar power. While there are a number of materials that can be used for the generation of energy from waste heat, they all suffer from various issues ranging from low stability to low efficiency. Nevertheless, the fact that a large number of industries generate copious amounts of waste heat have driven research into this field.
A team of scientists led by Professor Hiromichi Ohta at the Research Institute for Electronic Science (RIES), Hokkaido University, has recently developed a layered cobalt oxide with a record-setting thermoelectric figure of merit for metal oxides at room temperature.
Their findings were published in the journal Journal of Materials Chemistry A (“Layered cobalt oxide epitaxial films exhibiting thermoelectric ZT = 0.11 at room temperature”).
Thermoelectric conversion is driven by the Seebeck effect: when there is a temperature difference across a conducting material, an electric current is generated. Historically, the efficiency of heat-to-electricity conversion of metal oxides was very low; however, metal oxide-based thermoelectric devices are highly desired due to their environmental compatibility.
The thermoelectric conversion efficiency of a device depends on a key factor called the thermoelectric figure of merit (ZT).
Hiromichi Ohta’s group has developed a layered cobalt oxide that exhibits a high ZT and is stable across a range of operating temperatures. Well-known sodium-cobalt oxide, where sodium and cobalt oxide layers alternate, shows a very low ZT of around 0.03, but the material developed by Ohta’s group achieved a ZT of 0.11. The group replaced the sodium by other alkali or alkaline earth metals: calcium, strontium, and barium.

Correlation between the atomic mass and thermoelectric figure of merit (ZT). As the atomic
mass of the alkali or alkaline earth metal increases, the ZT also increases. Orange, calcium; yellow, sodium; purple, strontium; green, barium. (Image: Yugo Takashima et al,
Journal of Materials Chemistry A, October 13, 2020)
The layered barium-cobalt oxide material exhibited a record-setting ZT of 0.11 at room temperature. The increase in ZT is directly caused by the decreased thermal conductivity of barium. As the scientists hypothesized, the greater the atomic mass, the lower the thermal conductivity, resulting in higher ZT. This is due to the fact that heavier atoms suppress the vibrations in the cobalt oxide layers caused by heating.
Further research is required to optimize the material’s composition for higher efficacy and stability, as well as determining the most useful practical applications.
Source: Hokkaido University