The first information about artificial crystal growth from atomically-small metal nanoclusters (Researchers are learning to engineer the growth of nanometer-sized gold clusters) has been obtained in research conducted by researchers in Singapore, Saudi Arabia and Finland. The work was published in Nature Chemistry on November 10, 2022.
A normal solid is composed of atoms arranged in a crystal lattice. The chemical properties of atoms and lattice symmetry define the properties of substances, for example whether they are metals, semiconductors or electrical insulators.
Lattice symmetry can be changed by environmental conditions such as temperature or high pressure, which can change the structure and even change the electrical insulation in the electrical conductor, that is, metal.
Similar large objects such as nanoparticles or atomically broken nanoclusters can also organize themselves into crystal lattices, to create so-called meta-materials. However, information about how these objects grow from their cell walls is scarce, because crystal growth is a self-organizing process.
Today, the first information about the growth of crystals made from atomic-precision metal nanoclusters (Researchers are learning to engineer the growth of nanometer-sized gold clusters) was obtained in a study by researchers in Singapore, Saudi Arabia and Finland. They produced clusters of gold composed of just 25 gold atoms, one nanometer in diameter.
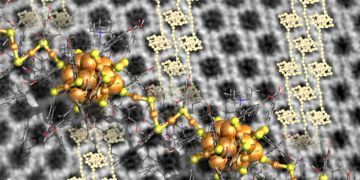
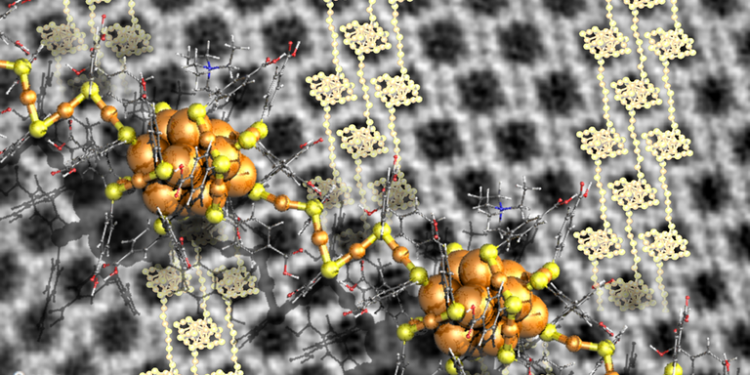
These clusters dissolve in water because of the ligand molecules that protect the gold. This cluster is known to self-assemble into well-defined compact crystals upon evaporation of the aqueous solvent.
However, the researcher came up with a new idea to improve crystal growth by adding tetra-alkyl-ammonium molecular ions to the solvent. These ions affect the surface chemistry of the gold cluster, and their size and concentration have been found to affect the size, shape, and texture of the crystals formed.
Remarkably, the high-resolution electron image of some crystals showed that they have polymer chains of clusters with links in the middle and three golds (see picture). The surface chemistry presented so far opens new avenues for the production of metal cluster-based meta-materials for the study of their electronic and optical properties.
Clustering was done at the National University of Singapore, electron microscopy was done at the King Abdullah University of Science and Technology in Saudi Arabia, and the study was done at the University of Singapore Jyväskylä, Finland.