Another crystal layer on top of the crystal acts as a precursor for crystal-to-crystal transformation. The surface of ice has a layer below its melting temperature of 0 ℃. Such a melting point is important for skating and Snowflake. Similarly, water tends to form thin crystals on a flat surface before it reaches the freezing temperature, i.e. before freezing. The thickness of the surface layer increases and changes as it approaches temperature changes (such as thawing and freezing). With the exception of thawing and pre-freezing, the existence of the surface as a factor that will cause changes in velocity is rarely investigated.
Han’s team at HKUST suggests that a polymorphic crystal layer may form on the surface of the crystal before the crystal-crystal phase transition and call it a solid phase transition. If the carbon atoms near the surface of the diamond can rearrange to form a graphite lattice before they reach the diamond-graphite transition, then it will be a so-called solid transition.
The process is essentially the same as melting or pre-freezing: the newly formed surface layer reduces the surface energy of the crystal. Han’s team pointed out that this is possible when two polymorphic crystals can form a uniform interface, that is, two lattices with the appropriate lattice spacing and the appropriate orientation at the interface.
Therefore, the surface of the denser polymorphic crystal can create a smaller crystal layer because the newly formed crystal-crystal interface is compact and loses almost all energy.
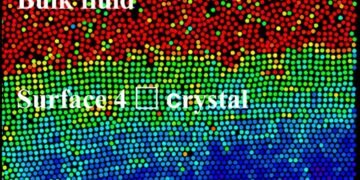
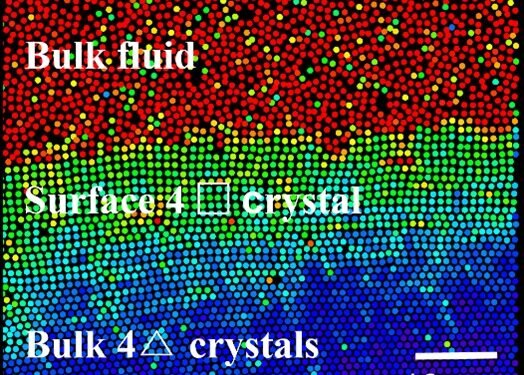
Han’s team also mentioned the presence of a strong exchange rate in experiments and computer simulations. They found that the surface of a thin colloidal crystal with a triangular lattice can form a square lattice because their interfaces are interconnected. The thickness of the surface layer increases with temperature as a power law similar to pre-melting.
This is also stated by their simulations on atoms with different interactions. Pre-melting was first invented by Michael Faraday, the father of electricity, in 1842, but it was not clearly experimentally confirmed until 1980. The second type of mouth, before freezing, was proposed and observed in the 1950-70s. The strong pre-stress movement proposed by Han’s team is a third type that brings water to the surface for seasonal changes.
Although this is a temperature phenomenon, they note that the crystal layer on the surface can also be in a non-equilibrium structure after sudden changes in temperature, such as melting, freezing and cooling. The surface layer makes the process easier and therefore can have advantages in the production and processing of materials.
Anomalous solid-state transitions that occur on the bare surface after passing through a solid-state transition point are ruled out by polycrystalline annealing, formalism, or other evidence. Also, they found a new liquid in the square lattice surface double layers and superimposed temperature regime of pre-melting and before the solid transition.
Colloids, such as milk, paint, blood, are usually liquid suspensions of small particles with uniform Brownian motion. Thermal trajectories can be followed under the microscope even in 3D masses of crystals or liquids, which are difficult to obtain in atomic systems. Therefore, colloids are used as solid model systems to measure chemical processes and time transitions.
“Our work shows that colloids are also effective in discovering new species, even for well-known traits in thermal processing.” Han said, “The process is simple, so it could have been proposed decades ago, but it seems to be a flaw in material science.
The future direction is to look for this phenomenon in atomic or molecular crystals. Unlike pre-melting which is common in most crystals, early solidification can occur in polymorphic crystals with low energy interfaces. We have proposed a number of candidates for atomic and molecular crystals with edged cavities.
Polymorphic crystals often have different properties, for example, graphite is soft, black and electrically conductive, while diamond is hard, which has a negative luster. A crystal with a polymorphic crystal layer will have very visible properties and such a layer can be regenerated after it has been formed or destroyed. Therefore, it should be very useful in the application.
Source: The Hong Kong University of Science and Technology (HKUST)