Breakthrough in Micelle Innovation for Compelling Color and Sedate Dispersion: Well-defined core-shell piece copolymer micelles make strides color solubilization and can clear the way for prevalent ink and color formulations.
Micelles are round atomic structures ordinarily shaped by amphiphilic particles with square structure, which contain both hydrophilic and hydrophobic parts. The hydrophobic tails of these particles cluster together to shape a center, whereas the hydrophilic heads confront outward, making a defensive shell. This structure permits micelles to typify hydrophobic substances inside their center and scatter them in a water-based environment.
An illustration of micelles in activity is in cleanser, which traps soil and oil, making them simple to flush off with water. Micelles can be made utilizing square copolymers, which have particular hydrophilic and hydrophobic portions, or irregular copolymers with a blended conveyance of hydrophilic and hydrophobic sections. The previous, utilized in the pharmaceutical industry, offers exact control over the micelle’s properties but is more complex and costly to deliver, whereas the last mentioned, utilized in the color industry, is less difficult and cheaper to produce.
Researchers driven by Mr. Masahiko Asada and Teacher Hidenori Otsuka from Tokyo College of Science (TUS) and DIC Enterprise, are exploring how to make micelles more viable at dissolving colors. In a ponder included on the cover of Volume 20, Issue 26 of the diary Delicate Matter distributed on July 14, 2024, they compared the execution of square copolymers and irregular copolymers to decide the most ideal micelle for color dispersion.
“There is a trade-off between utilizing irregular copolymers as dispersants for ink generation and their lacking scattering execution. We explored square copolymer micelles and compared their scattering execution with those of irregular copolymers to decide the micelle structure required for satisfactory color solubilization,” says Prof. Otsuka, the lead creator of the study.
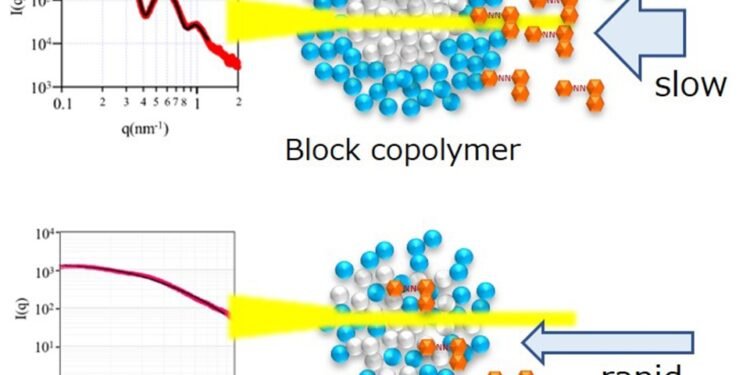
The analysts synthesized different square copolymers (BL01 to BL05) utilizing distinctive proportions of styrene (St), n-butylmethacrylate (BMA), and methacrylic corrosive (MA) as monomers. They compared the execution of these square copolymers with arbitrary copolymers (RD01, RD02, RD03, and RD04), which were made from styrene and either methacrylic corrosive or acrylic corrosive. The copolymers and arbitrary copolymers were scattered in water at a 0.5% concentration, and the micelle structures were inspected utilizing Little Point X-ray Diffusing (SAXS) analysis.
The SAXS comes about appeared that micelles shaped from square copolymers had a well-defined round structure with a clear core-shell boundary. Micelles from arbitrary copolymers were found to have a more diffuse and persistent structure, taking after a random-coil design with no unmistakable core-shell boundary. The nonattendance of a clear core-shell structure in the micelles shaped from irregular copolymers diminished their capacity to hold hydrophobic colors. In Basic Micelle Concentration (CMC) tests, the analysts measured the concentration at which micelles frame by recognizing changes in the extremity around a fluorescent pyrene test. The comes about appeared that square copolymer micelles had a much lower extremity, meaning the pyrene atoms were superior ensured interior the hydrophobic center of these micelles.
The analysts made comparative perceptions on measuring the degree of solubilization of hydrophobic orange oil SS color in the micelles. The micelles made utilizing arbitrary copolymers were found to let the color in effortlessly. In any case, BL01, BL03, and BL05 anticipated the color from entering the center, coming about in a longer time to reach immersion (2 days compared to 10 hours for the arbitrary copolymers). Micelles (BL01, 03, and 05) with bigger center volumes and more polymer atoms (higher accumulation numbers) were found to hold or solubilize more color (0.2 to 2 color atoms per micelle) than the littler micelles (BL02, BL04).
While the bigger micelles with well-defined core-shell structures took longer to gotten to be soaked with color, they seem hold a altogether higher sum of color. The micelle with the most elevated color solubilization was BL02. Its shell contained a irregular blend of methacrylic corrosive (MA) and butyl methacrylate (BMA), coming about in a exceedingly polydisperse or changed interface between the core-shell and shell-solvent boundaries, which permitted the color to enter and be removed from it quickly.
Prof. Otsuka clarifies, “The piece copolymer micelles displayed a higher color solubilization capacity, which connected with their center volume, clear core-shell differentiate, and moderate solubilization rate.” This finding may lead to more productive and cost-effective micelles for the ink and color businesses as well as pharmaceutical businesses.
Source: Tokyo University of Science