Intensive Chemotherapy Targets Brain Tumors Using New Ultrasound Technology, A device that uses microbubbles to open the blood-brain barrier to treat glioblastoma in humans
A major obstacle in treating the deadly brain cancer glioblastoma is that even the most powerful drugs cannot cross the brain barrier to reach the aggressive brain tumor.
And now, Northwestern Medicine scientists are reporting the results of a first-in-human clinical trial in which they used a new ultrasound device implanted in the skull to open the blood-brain barrier and bypass the area repeatedly, the brain is very dangerous intravenous chemotherapy.
A four-minute procedure is performed to open the blood-brain barrier and the patient is awake, and patients go home after a few hours. The results showed that the treatment was safe and well tolerated, with some patients receiving up to six treatments.
This is the first study to accurately determine the effect of opening the blood-brain barrier by ultrasound and the combination of chemotherapy in the human brain. Opening the blood-brain barrier leads to a four- to six-fold increase in drugs in the human brain, the results show.
Scientists have seen this increase with two different powerful chemotherapy drugs, paclitaxel and carboplatin. These patients are not treated with drugs because they do not cross the blood-brain barrier under normal conditions.
In addition, this is the first study to describe how quickly the brain barrier shuts after sonication. Most of the restoration of the blood-brain barrier occurs in the first 30 to 60 minutes after sonication, scientists discovered. The results will help improve drug delivery systems and use ultrasound to increase drug penetration into the human brain, the authors said.
“This could be a major breakthrough for glioblastoma patients,” said principal investigator Dr. Adam Sonabend, assistant professor of neurosurgery at Northwestern University Feinberg School of Medicine and neurosurgeon at Northwestern Medicine.
Temozolomide, the current chemotherapy used for glioblastoma, crosses the blood-brain barrier but is a weak drug, Sonabend said.
The article was published May 2 in The Lancet Oncology.
The blood-brain barrier is a visible substance that protects the brain from most circulating drugs. Therefore, the medical literature that can be used to treat brain diseases is very limited. Brain cancer patients cannot be treated with many drugs that are effective against cancer elsewhere in the body, because these do not cross the blood-brain barrier. Successful repurposing of drugs to treat brain diseases and cancer requires their delivery to the brain.
In the past, studies that injected paclitaxel directly into the brains of patients with these tumors showed promising signs of effectiveness, but direct injections were associated with toxicities such as brain damage and meningitis. , said Sonabend.
The blood-brain barrier closes after one hour
Scientists have found that using ultrasound and microbubble-based blood-brain barrier space is temporary and most of the blood-brain barrier is restored within one hour of this process in humans. Sonabend, who is a member of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University, said,
“There is a critical window of time after birth when the brain can access drugs that circulate in the bloodstream. Previous human studies have shown that the blood-brain barrier is fully restored 24 hours after brain sonication, and based on some animal studies, the field thinks that the blood-brain barrier is open for the first six hours and it is more. The Northwest study suggests that this window may be short.
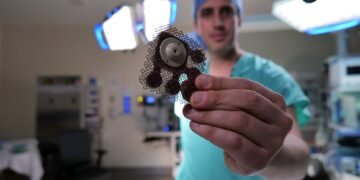
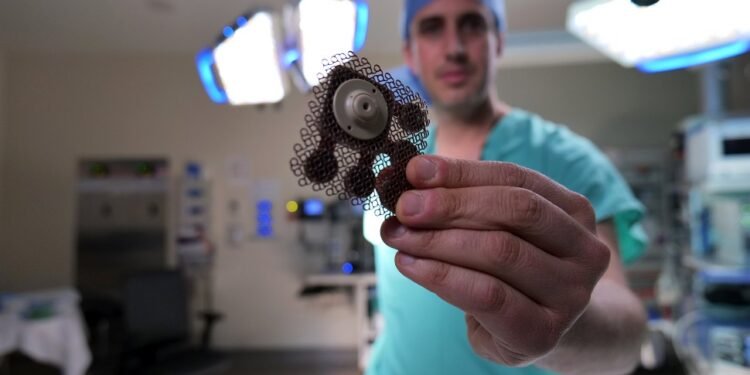
In another first, the study reports that the use of a new implantable grid in the skull of nine ultrasound transmitters made by the French biotechnology company Carthera opened the blood-brain barrier in the brain volume nine times larger than the original device (a small ultrasound-emitting implant) . This is important because in order to be effective, this method requires coverage of a large area of the brain near the cavity that remains in the brain after the removal of the glioblastoma. A clinical trial for patients with recurrent glioblastoma.
The results of this study are the basis of a second ongoing clinical trial led by scientists for patients with recurrent glioblastoma. The purpose of the trial – in which participants receive a combination of paclitaxel and carboplatin delivered to their brains through an ultrasound system – is to determine whether this treatment prolongs the life of these patients. A combination of these two drugs is used in other cancers, which is the basis of their association with the second process.
In Phase 1 of the clinic reported in this article, patients undergo surgery for the removal of their tumors and the insertion of an ultrasound device. They started treatment a few weeks after the injection.
Scientists increased the dose of paclitaxel given every three weeks and the introduction of ultrasound-based blood clots. In subsets of patients, studies were performed during surgery to investigate the effect of this ultrasound device on drug concentrations. The blood-brain barrier was visualized and mapped in the operating room using a fluorescent die called fluorescein and by MRI obtained after ultrasound therapy.
“Although we focus on brain cancer (there are about 30,000 gliomas in the United States), this opens the way to explore new drug treatments for millions of patients with various brain diseases. .” Sonabend said.
Source: Northwestern University