A team of researchers at the University of Texas MD Anderson Cancer Center has developed a Nanotechnology Platform Enables Reprogramming of Cancer Cells’ Immune System, Enabling Immunotherapy, that can change the way the immune system views solid tumor cells, making them more responsive to immunotherapy. Preclinical results show that this flexible mutation system can be easily applied to many types of cancer.


This study, published in Nature Nanotechnology, describes the use of this platform to involve molecules that act on tumor cells, triggering an immune response in vivo and in vitro. Wen Jiang, MD, Ph.D., assistant professor of radiation oncology, and professor of neurosurgery, are leading the study.
“With this new platform (Nanotechnology Platform Enables Reprogramming of Cancer Cells’ Immune System, Enabling Immunotherapy), we now have a plan to transform solid tumors, at least rare, into hematological tumors, which often have a high response to immunotherapy,” Jiang said. “If we can translate and validate this method in the clinic, it could bring us closer to the highest level of immunotherapy with cancer that has not yet been successfully treated.”
Immunotherapy has high response rates in blood cancers such as leukemia and lymphoma, but success has varied among solid tumors. Scientists have worked to better understand the mechanisms that restrict better responses. One explanation is that the differential expression of immune molecules in blood cancer and solid tumor cells affects how they interact with immune cells.
The activated lymphocyte signaling molecule 7 receptor (SLAMF7) is important for the activation of immune cells against cancer cells, acting as an “eat me” signal. However, it is found almost exclusively on the surface of blood cancer cells and not in solid tumor cells, making it an attractive target for translational therapy of researchers.
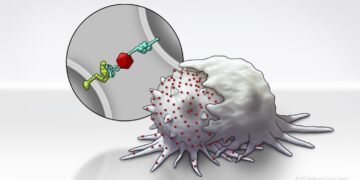
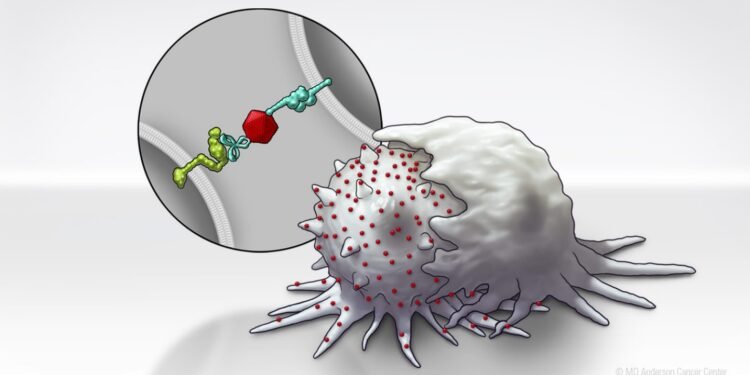
To enhance SLAMF7 expression in solid tumor cells, the researchers developed a Bispecific Tumor Transformation Nanoconjugate (BiTN) platform. These nanosystems are designed with one molecule to attach to the surface of targeted tumor cells and a second molecule to suppress the disease response.
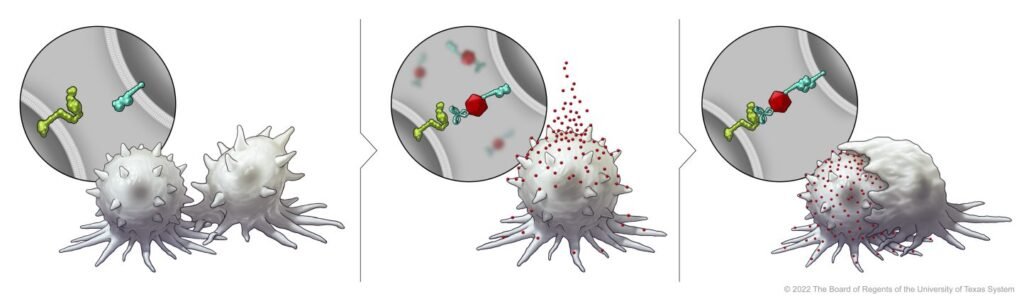
In this study, researchers used BiTN and SLAMF7 and HER2 antibodies to identify HER2-positive breast cancer cells. In a laboratory model, the nanoconjugate binds SLAMF7 effectively to breast cancer cells, triggering phagocytosis, or ingestion, by immune cells. The process also made breast cancer cells susceptible to treatment with anti-CD47 antibodies, which prevent tumor cells from “eating me” to mount more responses in solid tumors.
According to the authors, one of the most exciting aspects of this platform is its extensive application capabilities. This approach will not be cancer type or management specific, but may be a universal strategy for many different types of cancer. As a proof of concept, the authors also developed BiTN with folate instead of an anti-HER2 antibody to treat triple negative breast cancer with similar results.
“Because these materials are technologically engineered, this can be used as a scaffold to put different tumor-targeting or immune-fighting molecules on the surface of the nanoparticle. ,” Kim said. “For patients with solid tumors that don’t respond to immunotherapy, we see this as an added benefit to targeting the parts of the body that haven’t responded yet.”