The research team uses laser light to illuminate the interior of the planet’s ice – inspiring a new method for creating tiny diamonds.
What happens in planets like Neptune and Uranus? To find out, an international team of Helmholtz-Zentrum Dresden-Rossendorf (HZDR), the University of Rostock and the French École polytechnique conducted an unprecedented experiment. They fired a laser at a thin film of flat PET plastic and analyzed what happened using the intense laser beam. One of the results was that the researchers were able to confirm their earlier thesis that diamonds are indeed hidden in the ice sheets outside of our solar system. Another is that this process can establish a new way to create (New method for creating tiny diamonds) nanodiamonds, which are needed, for example, for quantum sensors with strong sensitivity.
The team presented their findings in the journal Science Advances (DOI: 10.1126/sciadv.abo0617).
The conditions inside giant planets like Neptune and Uranus are extreme: temperatures reach thousands of degrees Celsius and pressures are millions of times greater than Earth’s atmosphere. However, such states can be recreated very precisely in the laboratory: a powerful laser beam hits a film-like film, heats it up to 6,000 degrees Celsius and creates shock waves. which accumulates the material for a few nanoseconds. million times the atmosphere. “Until now, we used hydrocarbon films to do this type of experiment, explains Dominik Kraus, scientist at HZDR and professor at the University of Rostock. “We also found that this high pressure produced (New method for creating tiny diamonds) small diamonds, called nanodiamonds.”
Using these films, however, it is only partially possible to reproduce the contents of the planet, since the large ice sheets not only contain carbon and hydrogen, but also large amounts of oxygen. While looking for a suitable film, the group found something that happens every day: PET, the resin from which ordinary plastic bottles are made. “PET has the right balance of carbon, hydrogen and oxygen to make the planet’s ice work,” says Kraus.
The team conducted their experiments at the SLAC National Accelerator Laboratory in California, home of the Linac Coherent Light Source (LCLS), a fast X-ray based laser. They used it to investigate what happens when a strong laser beam hits PET film, using two measurement methods at the same time: X-ray diffraction to determine if nanodiamonds have been formed, and a so-called small advertisement. A big diamond has grown.
A big help: oxygen
“The effect of oxygen is to accelerate the separation of carbon and hydrogen and thus encourage the formation of nanodiamonds,” explains Dominik Kraus, reporting the results. “This means that carbon atoms can easily combine and form diamonds.”
This also supports the theory that it is actually a rain of diamonds in a large ice sheet. This discovery is possible not only for Uranus and Neptune, but also for many other planets in our galaxy. Although these ice giants were once considered rare, it now seems clear that they may be a common species outside the solar system.The group also encountered a demonstration of another type: in combination with diamonds, water will be produced – but in different forms. “This so-called superionic liquid can form,” Kraus said. “Oxygen atoms form a crystal lattice in which hydrogen atoms move freely.” Since it is electrically charged, superionic water can conduct electricity and thus help create the magnetic fields of ice giants.
In their experiments, however, the same research has not been able to clearly show the existence of superionic water in combination with diamonds. This should be done in collaboration with the University of Rostock and the European XFEL in Hamburg, the most powerful X-ray laser in the world. HZDR leads the HIBEF international team there, which provides a good environment for such experiments.
The right tree for nanodiamonds
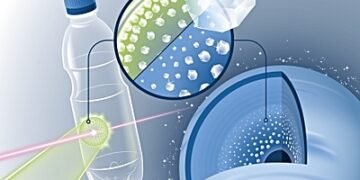
In addition to this basic knowledge, new experiences also open up prospects for a technical application: cloth-made production of nano-sized diamonds, which are already included in abrasives and polishing agents.In the future, it is expected that they will be used as critical quantum sensors, health monitors and positive reaction accelerators, for example to separate CO2. “Until now, diamonds of this type were produced by explosives,” says Kraus. “With the help of laser light, it is possible to make them even cleaner in the future.”
The vision of the scientists: A high-performance laser light fires ten times per second and the PET film of the light shines in the center of the tenth of a second. Nanodiamonds thus create a film from the film and enter a collection tank filled with water. There, they are easily broken down and can be cleaned and supplied properly.The important advantage of this method compared to the production of explosives is that “nanodiamonds can be cut to measure the size or even be attached to other atoms”, emphasizes Dominik Kraus. “The X-ray laser means we have a laboratory tool that can control the growth of diamonds.”