Perovskite halide materials exhibit atomic vibrations similar to liquids, Halide perovskites are a recently developed type of material. They have applications in solar energy and radiation detection. They can also be useful for heat recovery, taking heat from engines and other sources.
Cesium lead bromide (CsPbBr3) is one of the simplest members of the large family of halogenated perovskites. Understanding the atomic structure and energy of Lead Halide Perovskites (LHPs) is important for scientists developing these materials for applications. LHPs exhibit structural stability and large atomic fluctuations that scientists believe affect their optics and light. However, scientists do not fully understand the motion of atoms in the LHP.
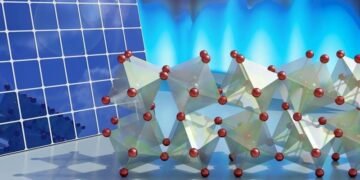
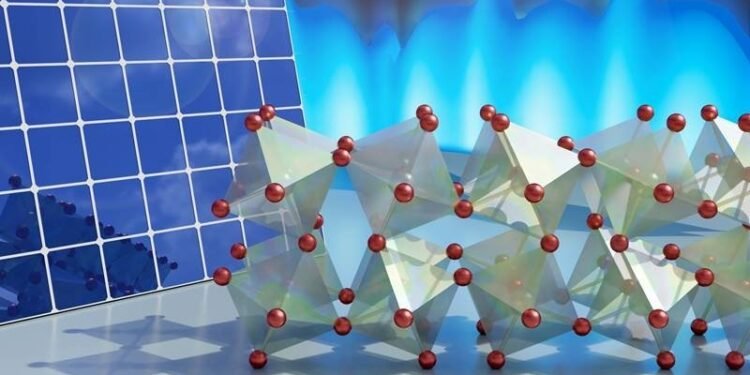
In this study, scientists used neutron and X-ray scattering using computers to reveal the unique behavior of coordinated atomic motion at the LHP. Specifically, the researchers found that the atomic vibrations (phonons) of bromine octahedra (see picture) have large amplitudes but cannot be repeated for a long time. Instead, the vibration is very disruptive. It’s like when a guitarist puts his palm on the strings of the instrument as he strums them, making a sound.
The atomic vibrations in the LHP can help the electrons produced by photon extraction to travel long distances in the device. This will cause the material to produce electrical energy. This research opens up new ways to design materials with optical and thermal behavior.
These materials have many potential applications. For example, they can adapt solar panel technology and heat recovery equipment. In these applications, halide perovskites can lead to devices that are efficient, cheap and easy to manufacture.
Understanding the interaction of phonons and electronic states of freedom is important for the control of many energy sources. Cesium lead bromide is an interesting material that exhibits a complex atomic structure and is the simplest structure of a large family of halide perovskites for photovoltaics, radiation detection, and light source applications.
This research investigated the dynamics of strong anharmonic phonons in these materials by combining neutron scattering and X-ray experiments at the Spallation Neutron Source and the Advanced Photon Source, both facilities of the Department of Energy Office of Science.
The team included researchers from Duke University, Argonne National Laboratory, Oak Ridge National Laboratory, National Institute of Standards and Technology, University of Maryland and Northwestern University.
The researchers’ analysis of the experimental results, combined with computer simulations, established that strong anharmonic lattice vibrations (that is, vibrations with frequencies that are not well defined and that do not last for a long time) are associated with unsteady support oscillations of bromine octahedra in the crystal structure. , as shown in the picture above.
These charges change the electronic state near the band gap and are believed to disrupt the combination of excited photo-charges. Therefore, anharmonic phonons and electron-phonon coupling can be the key to high performance in photovoltaic and radiation sensing applications.
A new understanding of crystal lattice atomic vibrations provides new avenues for designing materials with optoelectronic and thermal control for future energy harvesting and conversion devices.
Source: U.S. Department of Energy