A team from Lawrence Berkeley National Laboratory (Berkeley Lab) and Florida State University has developed a new prototype for a solid state battery that does not rely on specific chemical materials, especially metals that are difficult to find due to supply problems. Their work, recently described in the journal Science, could improve solid-state batteries efficiently and cheaply.
Known for their high power and high safety, solid state batteries could be a game changer for the electric vehicle industry. But developing cheap and efficient enough to make a car run hundreds of miles on a single charge has been a tough hurdle to overcome.

“Our work is the first to solve this problem by creating a strong electrolyte that is not only a single metal, but also a group of cheap metals,” said first author Yan Zeng, a scientist at the Department of Materials Science. property, said by Berkeley Lab.
In a lithium-ion battery, the electrolyte acts as a transfer point where the lithium ions travel with an electrical charge to power the device or charge the battery.
Like other batteries, solid state batteries store energy and release it to power devices. But instead of liquid or gel polymer electrolytes in lithium-ion batteries, they use a solid electrolyte.
Governments, research and academia have invested heavily in the research and development of solid state batteries because the liquid electrolytes used in most commercial batteries are prone to overheating, overcharging and overcharging.
However, most of the powerful batteries that have been built so far rely on a variety of expensive metals and not in large quantities. Some are not in the United States at all.
For the current study, Zeng – along with Bin Ouyang, an assistant professor of chemistry and biochemistry at Florida State University – and lead author Gerbrand Ceder, a research director at Berkeley Lab and a professor of materials science and engineering at UC Berkeley presented the model new. strong electrolyte consists of a mixture of different whites. Zeng and Ouyang first developed the idea for this project while completing postdoctoral research at Berkeley Lab at UC Berkeley under Ceder’s supervision.
This new material can make the electrolyte more conductive than the one that does not depend on the large amount of one person. In experiments at Berkeley Lab and UC Berkeley, researchers demonstrated a new solid electrolyte by creating and testing several lithium-ion and sodium-ion materials with several mixed metals.
They found that the new multi-metal material performed better than expected, exhibiting ionic conductivity several orders of magnitude better than single-metal materials. Ion conductivity is a measure of how quickly lithium ions conduct electricity.
The researchers think that mixing many different types of metals creates a new way – like adding a road to a highway – in which lithium ions can pass more easily through the electrolyte. Without these methods, the movement of lithium ions will be slow and prevent them from going through the electrolyte from one part of the battery to another, Zeng explained.
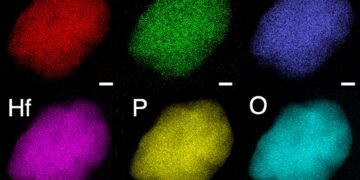
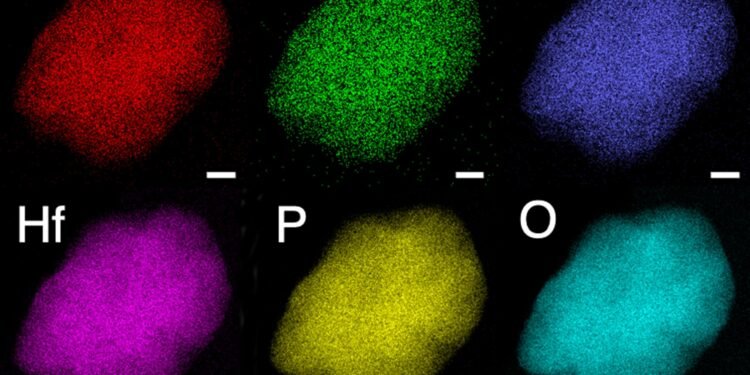
To confirm the candidates for the multi-metal design, the researchers performed advanced theoretical calculations based on a method called density functional theory on supercomputers at the National Energy Research Scientific Computing Center (NERSC). Using transmission electron microscopes (STEM) at the Molecular Foundry, researchers have confirmed that each type of electrolyte – what scientists call a “single phase” – bends in an unusual pattern. leading to new ion transport pathways in it Crystal structure.
This discovery opens up new opportunities to design next-generation ionic conductors. The next step in this research is to apply the new method Zeng developed at Ceder and Berkeley Lab to identify and identify new solid electrolytes that can improve battery performance.
This project represents one of the many ways Berkeley Lab Energy Storage Center experts are working to facilitate the nation’s transition to a clean, affordable and sustainable energy landscape.
Last year, Ouyang won the NERSC High Performance Computing Achievement Award for “improving the understanding of short-term chemical control for creating a new generation of commercial cathode materials.” This award recognizes early-stage scientists who have made significant contributions to scientific computing using NERSC resources.