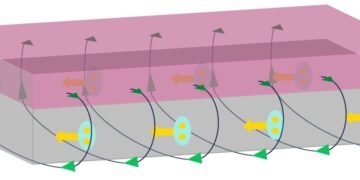
University of California, Berkeley, chemists have created a new type of material (The new “chainmail” of interconnected molecules is strong, flexible and easy to create) from millions of interacting molecules that allows for the first time the synthesis of extended 2D or 3D structures that change flexible, strong and durable, like chain mail preserved ancient times knight.
A so-called infinite catenane, can be created in one chemical step.
French chemist Jean-Pierre Sauvage shared the 2016 Nobel Prize in Chemistry for the creation of the first catenane – two linked rings. These structures serve as the basis for the creation of transportable molecular structures, commonly referred to as machines.
But the chemical structure of catenan remains active. Adding each ring to the catenane requires another chemical reaction. In the 24 years since Sauvage developed the two-ringed catenane, chemists have made, or rather, only 130 rings linked together in quantities too small to see without an electron microscope.
The new type of catenane, developed in the laboratory of UC Berkeley chemistry professor Omar Yaghi, can be created in any number of three-dimensional structures. Since each individual piece is mechanically bonded without chemical bonds, the structure can be flexed without breaking.
Yaghi, James and Neeltje said, “We think that has a lot of implications, not only in creating strong things that don’t break, but also things that will go into robotics and aerospace and armored suits and things like that.” like that,” said Yaghi, James and Neeltje. Tretter Professor of Chemistry, Director of BASF’s Kavli Energy NanoSciences Institute and California Research Alliance, and Principal Scientist of UC Berkeley’s Bakar Institute of Digital Materials for the Planet.
Yaghi and his colleagues, including first author Tianqiong Ma, a UC Berkeley postdoctoral fellow, reported details of the chemical process this week in the journal Nature Synthesis.
Reticular Chemistry
The breakthrough in catenane production is possible thanks to a type of chemistry that Yaghi invented more than 30 years ago: reticular chemistry. He describes it as “an arrangement of molecular blocks in a crystal structure extended by strong bonds”.
Using this method, he has created inexpensive materials – metalorganic frameworks (MOFs) and covalent organic frameworks (COFs) – which are useful for capturing, storing or separating gases such as carbon dioxide, hydrogen and water vapour. More than 100,000 types of MOFs have been developed to date.
To make MOFs, all you have to do is synthesize food hybrid molecules – metal clusters connected to organic ligands – and mix them in a solution so that they join together to form a rigid, highly porous 3D network. One chemical in the process is chosen to create and release – depending on the temperature – different molecules and reject others.
The MOF created by Yaghi can remove water from even dry air and release it when it’s hot, allowing water to be captured in deserts.
To make catenans, Yaghi and Ma created a molecule with a cross between two parallel halves, held together by copper atoms. The structure, what they call catena-COF, is reminiscent of two boomerangs connected by copper atoms where they collide. When mixed together, these molecules bond together to form a 3D network of building blocks. The building block, a polyhedral molecule called adamantane, joins its six arms to form an extended center.
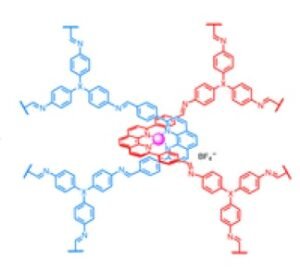
“What is new here is that the houses that are being built have these crossovers, and because of the crossovers, you get an interlocking system that has interesting, flexible and resilient properties,” Yaghi said. “They are scheduled to meet in one step. This is the power of lattice chemistry. Instead of building them one piece at a time to create a larger structure, you’ve designed them to fit together and grow on their own.
A molecule with a crossover can be changed chemically so that the final catenan interacts with certain compounds. Yaghi calls these (∞) catenans, using the infinity symbol.
“I think this is the first step in creating things that can be moved and can be stiff in response to stimuli, like a movement,” he said. “So in some areas it can be flexible, and in some other areas it can become very difficult, just because of the way the work is done.”
He said that if these catenans extend three-dimensionally at the microscopic level, they could be thin enough for two-dimensional use, such as clothing. Recently, some scientists report that they have created MOF and COF through 3D printing, so it is possible to 3D print catenans, like weaving.
“Traditionally, this palm is made through a series of tedious steps to make only molecules with one or two or three interconnected rings, or polyhedra. But using do things that have amazing properties, like strength and stability, you have to do millions and millions of these things together,” he said. “The traditional way of making them is not enough. And lattice chemistry comes into the building blocks and finds a way to do it in one step. That’s really the strength of this story.